Course
Arterial Blood Gas Analysis
Course Highlights
- In this Arterial Blood Gas Analysis course, we will learn about the physiological basis of arterial blood gases.
- You’ll also learn signs and symptoms of acid-base imbalances and respiratory disturbances.
- You’ll leave this course with a broader understanding of ABG analysis to manage patient care effectively in both emergency and routine healthcare settings.
About
Contact Hours Awarded: 3
Course By:
Abbie Schmitt, MSN-Ed, RN
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction
It is such a powerful tool to understand and master one of the most insightful topics in clinical nursing practice—Arterial Blood Gases (ABGs)! Imagine you are a detective in a medical mystery. Your tools? The ABG results. Your mission? To understand the results and evaluate your patient’s respiratory and metabolic status. Like Sherlock Holmes with his magnifying glass, ABGs allow you to look closer at the clues hidden in the bloodstream, clues that can mean the difference between stability and crisis.
Let’s get pumped (with oxygenated blood) and ready to dive into the amazing world of Arterial Blood Gases!

What is Arterial Blood Gas?
Arterial blood gas tests, or ABGs for short, are the laboratory tests that measure the acidity, or pH, and the levels of oxygen (O2) and carbon dioxide (CO2) from arterial blood. It assesses the function of the lungs and how well they can move oxygen into the blood and remove carbon dioxide.
An ABG is one of the most commonly used tests to measure oxygenation and blood acid levels. It’s commonly performed in the ICU and ER setting; however, ABGs can be drawn on any patient on any unit, depending on their diagnosis.
Various parameters can measure the level or adequacy of oxygen in the blood.
ABGs provide the healthcare team with valuable insights into a patient’s condition and the effects of the team’s efforts. However, the interpretation of an ABG is a fairly complicated process that can be challenging to understand.
Before we learn how to analyze an ABG, it’s important to understand what exactly it is, the different components, and their corresponding ABG values.
The major components of an ABG are pH, bicarbonate, carbon dioxide, and oxygen.

Self Quiz
Ask yourself...
- Are ABGs commonly used in the field/unit that you work in?
- Can you name the components of the ABG assessment?
- What types of tools can be used to measure oxygen levels in the blood?
- Do you think it is important to memorize normal laboratory levels for ABGs?
Indications for ABGs
ABGs are obtained for a variety of clinical concerns.
Indications may include the following:
- Acute respiratory distress syndrome (ARDS)
- Sepsis
- Hypovolemic shock
- Diabetic ketoacidosis (DKA)
- Asthma
- Chronic Obstructive Pulmonary Disease (COPD)
- Pneumonia
- Emphysema
- Acute heart failure
- Cardiac arrest
- Renal tubular acidosis (RTA)
- Kidney Failure
- Trauma
- Chronic vomiting
- Uncontrolled diabetes
- Drug Overdose
- Metabolic Disease
- Chemical Poisoning
Components of ABGs
- PH
- PaCO2 (Partial Pressure of Carbon Dioxide)
- PaO2 (Partial Pressure of Oxygen)
- SO2 (Oxygen Saturation)
- HCO3- (Bicarbonate)
- BE (Base Excess)
pH
The pH is the concentration of hydrogen ions. It essentially determines the acidity or alkalinity of body fluids. A pH of 7.35 indicates acidosis and a pH greater than 7.45 indicates alkalosis.
There’s an inverse relationship between pH and H+, meaning that when there’s more H+, the pH decreases and becomes more acidic, and, when there’s less H+, the pH increases, and becomes less acidic.
If the pH is less than 7.35, it’s considered acidosis, while a pH greater than 7.45 is alkalosis.
The normal pH ranges from 7.35 to 7.45mmHg.
PaCO2 (Partial Pressure of Carbon Dioxide)
PaCO2 represents the adequacy of the gas exchange between the alveoli and the external environment.
Carbon dioxide is a gas, and gases release pressure. Carbon dioxide (CO2) becomes trapped when there is damage in the alveoli, and this excess of CO2 pressure combines with water to form carbonic acid (H2CO3), causing an acidotic state.
If hypoventilation occurs at the alveolar level (for example, in COPD), the PaCO2 is elevated and results in respiratory acidosis. If hyperventilation occurs at the alveolar level, the PaCO2 is decreased causing respiratory alkalosis.
The normal PaCO2 should range from 35 to 45mm Hg.
PaO2 (Partial Pressure of Oxygen)
PaO2 is the amount of oxygen available to bind with hemoglobin. The pH plays a role in the combining power of oxygen with hemoglobin: a low pH means there is less oxygen in the hemoglobin. The “p” stands for partial pressure, and “a” stands for arterial (4).
PaO2 measures the amount of carbon dioxide dissolved in the arterial blood; and partial pressure of oxygen, or PaO2, which measures the amount of oxygen in the arterial blood.
There is a common misconception that PaO2 is lowered in anemia; however, PaO2 is independent of hemoglobin and can be normal even in the presence of anemia. In anemia, however, it is the oxygen content (CaO2) that is lowered. (4)
Carbon monoxide (CO) poisoning is another serious situation where PaO2 can be normal. In clinically evaluating suspected poisoning, the percentage of carboxyhemoglobin in the arterial blood should be measured. Modern ABG machines routinely measure the percentage of carboxyhemoglobin.
Even when the oxygen content of blood is normal, oxygen may not be transported to tissues if the heart is not pumping efficiently. The heart must have sufficient cardiac output to deliver the blood to the peripheries.
The normal range for PaO2 is 75 to 100 mmHg.
SO2 (Oxygen Saturation)
SO2 is the amount of oxygen in the blood that combines with hemoglobin. It can be measured indirectly by calculating the PAO2 and pH or measured directly by oximetry.
Oxygen saturation is the percentage of hemoglobin that is combined with Oxygen. Normally, more than 95% of hemoglobin is combined with Oxygen in the arterial blood (4).
Ways to Measure Saturation
- Placing an Oximeter probe on body parts such as fingers, ear lobes, toes, etc.
- This is called as SpO2. The p in the SpO2 stands for peripheral).
- Taking a blood sample directly from an arterial puncture
- The blood gas machine then can measure the Oxygen saturation. This is called SaO2. The a in SaO2 stands for arterial.
- A blood gas analyzer is used.
In certain instances, the Oxygen saturation can be measured on venous or capillary blood.
The SpO2 may not be reliable for the following circumstances:
- Conditions when peripheral circulation is compromised.
- Examples: Peripheral vascular disease and shock.
- Black melanotic skin
- Excessive blood bilirubin in blood (e.g. jaundice) may give falsely low SpO2 levels.
- Black Henna tattoos may give falsely low SpO2 levels.
- The relationship between SpO2 and PaO2 is not linear.
- Even when SpO2 or SaO2 is around 90%, the actual PaO2 can be very low.
- The Oxygen dissociation curve is sigmoid-shaped
- Stressful situations such as hypoxia, acidosis, or increased body temperature can cause the hemoglobin to release more Oxygen to the tissues. However, in opposite conditions like alkalosis and hypothermia, the hemoglobin releases less Oxygen to the tissues.
HC03- (Bicarbonate)
HCO3-, or bicarbonate ion, is an alkaline substance that contains more than half of the total buffer base in the blood. Bicarbonate, HCO3- is a base. A base is a substance that can join with H+ and remove it from the solution. (2)
If HCO3- increases, the pH increases and becomes more basic, but if there is less HCO3-, the pH decreases and becomes more acidic.
A deficit of bicarbonate indicates metabolic acidosis; when there is an increase in bicarbonates, the result is metabolic alkalosis. (4)
HCO3- normally ranges from 21 to 28 mEq/L.
BE (Base Excess)
BE. Base excess or BE value is routinely checked with HCO3 A base excess of less than –2 is acidosis and greater than +2 is alkalosis. Base excess, the normal range is –2 to +2 mmol/L
PiO2 (Partial Pressure of Oxygen in Inspired air)
Oxygen is a gas, and gases exert partial pressure. The PiO2 is a partial pressure of Oxygen in Inspired air. During inspiration, as the Oxygen passes from the air into the blood, the partial pressure continues to decrease. Oxygen comprises 21%, i.e. 0.21 (approximately) of air.

Self Quiz
Ask yourself...
- Can you define pH?
- What are the normal values for pH, S02, and PaCO2?
- How would you describe a base?
- In what circumstances would SpO2 not be a reliable method to assess oxygen saturation?
Pathophysiology of Control of Acid-Base Balance
Our body’s balance between acidity and alkalinity is referred to as acid-base balance.
The acid-base balance is delicate and even a minor deviation from the normal range can severely affect many organs. There are different mechanisms used by the body to control the blood’s acid-base balance.
These mechanisms involve the:
- Lungs
- Kidneys
- Buffer systems
Role of the Lungs
Compensation and control of pH involves the release of carbon dioxide from the lungs. Carbon dioxide, which is mildly acidic, is a waste product of the metabolism of oxygen and cellular nutrients. It then passes from the cells into the blood. The blood carries carbon dioxide to the lungs, where it is exhaled. As carbon dioxide accumulates in the blood, the pH of the blood decreases (acidity increases).
The brain regulates the amount of carbon dioxide that is exhaled by controlling the speed and depth of breathing (ventilation). The amount of carbon dioxide exhaled, and consequently, the pH of the blood increases as breathing becomes faster and deeper. By adjusting the speed and depth of breathing, the brain and lungs are able to regulate the blood pH minute by minute.
Role of the Kidneys
The kidneys can affect blood pH by excreting excess acids or bases. The kidneys make these adjustments more slowly than the lungs do and compensation generally takes several days.
Buffer Systems
Another mechanism for controlling blood pH is the use of chemical buffer systems. These buffer systems protect against sudden shifts in acidity and alkalinity with the use of the body’s own naturally occurring weak acids and weak bases. These weak acids and bases minimize changes in the pH of a solution by adjusting the proportion of acid and base.
The most important pH buffer system in the blood:
- Carbonic acid
-
- A weak acid byproduct created from the carbon dioxide dissolved in the blood
- Bicarbonate ions
-
- The corresponding weak base
The Difference in Oxygen Content of Arterial and Venous Blood
Oxygen (O2) is delivered to tissues by the capillaries from the arterial blood supply. Oxygen is pulled into tissue and blood is returned by the veins. The amount of oxygen in arterial blood is higher than that of venous blood.
If the oxygen content of venous blood is subtracted from the oxygen content of arterial blood, it would provide a good idea of whether the tissues are extracting the appropriate amount of oxygen or not.
In certain conditions, the tissues may not fully utilize oxygen even if the cardiac output, hemoglobin, CaO2, and PaO2 are normal. The O2 is delivered to the tissue, but it is unable to extract the O2, and the unused oxygen is returned to the veins. This elevates the O2 of blood in the veins.
In conditions of lack of oxygen, tissues use anaerobic pathways. Anaerobic metabolism generates an excess amount of lactic acid (lactate). The blood lactate level can be used as an indicator of tissue hypoxia.

Self Quiz
Ask yourself...
- How do the lungs compensate for or correct abnormalities in pH?
- How do the kidneys impact the levels of pH?
- Can you describe the mechanisms of chemical buffer systems within the body?
- Why is it important to recognize the difference in arterial and venous blood supply when considering ABGs?
Interpreting Arterial Blood Gas Imbalances
Understanding the mystery of ABG analysis begins with knowledge of normal ranges and recognizing the signs and outcomes imbalances.
Normal ABG Values
It is imperative to know the normal values to recognize what deviates from normal. Memorize these values.
The normal ABG values are the following:
- pH: 7.35 to 7.45
- PaCO2: 35 to 45 mmHg (respiratory determinant)
- PaO2: 75 to 100 mmHg
- HCO3: 22 to 26 mEq/L (metabolic determinant)
- Oxygen saturation (02), 94–100%
- Base excess, the normal range is –2 to +2 mmol/L
These values may differ slightly depending on the laboratory.
Goals of Analysis
There are many different methods to help remember how to analyze arterial blood gas. It’s a nurse’s responsibility to be able to identify key components and be prepared for the next step. It can also be helpful to ask more experienced clinical nurses and respiratory therapists if you feel unsure about this process.
The goals that we need to accomplish when interpreting arterial blood gases include determining the following:
- Acidosis or Alkalosis
- Metabolic or Respiratory
- Fully Compensated, Partially Compensated, or Uncompensated
The normal range for ABGs is used as a guide, and the determination of disorders is often based on the blood pH level, which represents the acid-base balance.
There are two abnormalities of acid-base balance:
- Acidosis: The blood has too much acid (or too little base), resulting in a decrease in blood pH.
- Alkalosis: The blood has too much base (or too little acid), increasing blood pH.
If the blood has an excess base, the HCO3 level is evaluated because the kidneys regulate bicarbonate ion levels.
If the blood is acidic, the PaCO2 is assessed because the lungs regulate the majority of acid.
The categories to recognize in analyzing ABGs are:
- Respiratory Acidosis
- Respiratory Alkalosis
- Metabolic Acidosis
- Metabolic Alkalosis


Self Quiz
Ask yourself...
- What are the three goals of ABG analysis?
- Are you familiar with senior nurses or respiratory therapists within your organization who would be a helpful resource in cases of ABG irregularities?
- Which body organ regulates bicarbonate ion levels?
- Which body organ is an important part of regulating acid levels?
Compensation for Acid-Base Disorders
Compensation is the body’s attempt to maintain a normal pH level.
The carbon dioxide level is controlled by the respiratory system, and the renal system controls the bicarbonate level. These two systems are used to oppose each other to maintain a normal pH (7).
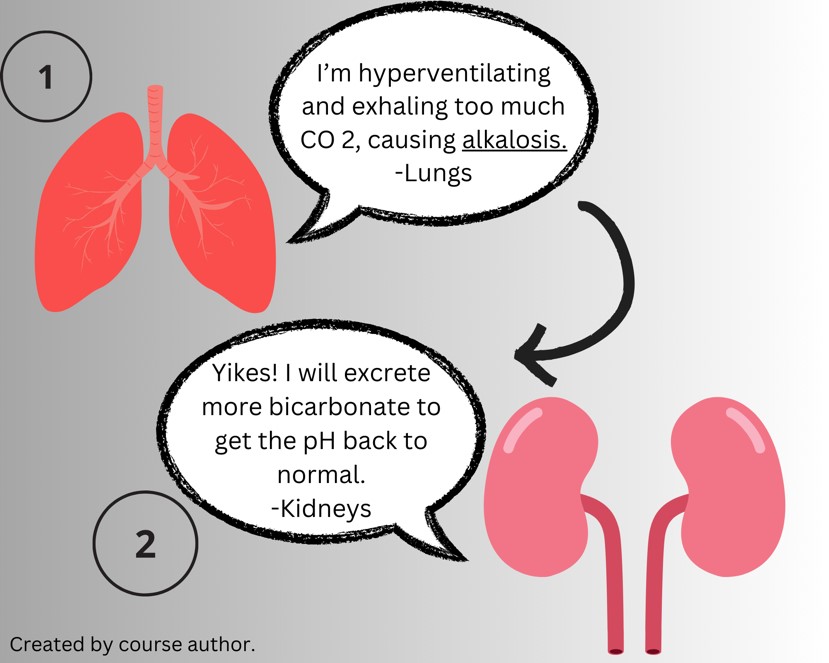
There are three categories of compensation. Uncompensated imbalance means the respiratory or renal system has not attempted to compensate for the changing pH. A partially compensated state means the opposing body system is attempting to compensate but has not successfully brought the pH back to normal. Fully compensated happens when the pH is within normal limits and values for the respiratory and metabolic components outside their normal ranges but in opposite directions (7).
An example is a patient who is rapidly breathing and exhales too much carbon dioxide, which lowers their PaCO 2 and increases the pH of arterial blood. The body tries to compensate for this alkalosis by excreting more bicarbonate from the kidneys and making arterial blood more acidic.
Initially, the compensatory mechanisms may restore the pH to nearly back to normal. Thus, if the blood pH has changed significantly, it means that the body’s ability to compensate is failing. In such cases, the underlying cause of the acid-base disturbance is urgently sought.

Self Quiz
Ask yourself...
- What would a significant change in pH signify?
- Which disturbances does the respiratory system compensate for?
- Which disturbance does the renal system compensate for?
- Would hyperventilation likely lead to acidosis or alkalosis?
Case Study: A Systematic Approach to ABG Analysis
You receive the following ABG results:
pH: 7.50
PaCO2: 31 mm Hg;
HCO3-: 24 mEq/L
PaO2: 89 mm Hg
SaO2: 98%.
The pH is high, the PaCO 2 is low. All other values are within normal limits.
These values should be assessed in the following steps (3):
- Step 1: Evaluate the PaO2 and the SaO2 levels for hypoxemia and intervene if necessary. There is no hypoxia present, these levels are normal. Continue to monitor the patient’s oxygenation status.
- Step 2: Look at the pH. Is it acidic or alkaline? In the example, the pH of 7.50 indicates alkaline.
- Step 3: Look at the PaCO2. Is it acidosis or alkalosis? In this example, the PaCO 2 is low. The respiratory component indicates alkalosis.
- Step 4: Examine the HCO 3 -. Is it acidosis or alkalosis? In the example, this metabolic component is normal.
- Step 5: Is the acid-base disturbance respiratory or metabolic? In this example, the low PaCO2 matches the high pH, indicating respiratory alkalosis.
- Step 6: What is the compensation? In the example, the patient would be partially compensated if this value had been outside the normal limits on the acidic side and the pH was still outside normal limits.
- Step 7: This patient has an uncompensated respiratory alkalosis with normal oxygenation.
Respiratory Alkalosis
When the pH of arterial blood is more than 7.45, it is considered alkalemia. Alkalosis refers to a broader context of tissue. These terms are often used interchangeably.
Respiratory alkalosis occurs when the primary abnormality is a decreased level of PaCO2.
In respiratory alkalosis, the decreased PaCO2 is the loss of CO2 from hyperventilation, which is caused by increased rate and/or depth of respiration (5). Decreased PaCO2 can occur in any type of hyperventilation.
Hyperventilation can occur in any condition that results in stimulation of the respiratory center.
Tachypnea clears or excretes the CO2, which results in respiratory alkalosis.
Causes of Respiratory Alkalosis
As mentioned, any condition that leads to stimulation of the respiratory center and an increased respiratory rate (tachypnoea) can cause respiratory alkalosis.
Examples of conditions that lead to stimulation of the respiratory center include (5):
- Lung Disorders
- Hypoxia can increase the respiratory rate. Examples include asthma, pneumonia, and pulmonary embolism Fever
- Drugs which stimulate respiration are catecholamines, vasopressor agents, salicylates, medroxyprogesterone, etc.
- Poisoning with stimulants such as amphetamines, salicylate poisoning, etc.
- Brain disorders such as brain stem encephalitis, multiple sclerosis
- Trauma to the brain
- Chest wall trauma and fibrosis can lead to tachypnoea
- Psychological distress, anxiety, and panic attacks
- Pain can cause hyperventilation.
- Hyperventilation of a mechanically ventilated patient
- Recovery from metabolic acidosis
In response, the body systems try to compensate for respiratory alkalosis by lowering blood bicarbonate levels by increasing excretion by the kidneys.
PaCO2 and HCO3 can move in the same direction in both respiratory acidosis and respiratory alkalosis. Essentially, they can both increase and decrease.
However, if these levels progress in opposite directions, this may indicate a more complicated disorder (5).
Clinical Findings in Respiratory Alkalosis
Decreased carbon dioxide causes vasoconstriction.
Alkalosis can cause metabolic abnormalities such as hypokalemia and hypocalcemia.
These abnormalities may lead to clinical features related to cardiovascular and neurological abnormalities.
- Cardiovascular Features:
- Peripheral vasoconstriction
- Angina
- Myocardial ischemia
- Arrhythmias.
- Neurological Features:
- Cerebral vasoconstriction and decreased intracranial pressure.
- Lightheadedness
- Confusion
- Neuromuscular irritability: Tetany, muscle cramps, Chvostek’s sign, Trousseau’s sign.
- Paranesthesia in the extremities.
- Note: Respiratory alkalosis can commonly be misdiagnosed as peripheral neuropathy.
- Seizures
The cerebral vasoconstriction can lead to transient ischemic attacks and strokes (5).
Management of Respiratory Alkalosis
As discussed previously, severe alkalosis can lead to cardiac and neurological complications in susceptible individuals. It is recommended to treat acute alkalosis when a patient has hemodynamic instability, altered mental status, and cardiac arrhythmias. Treatment is recommended particularly when the pH is more than 7.55.
Treatment methods goals include:
- Decreasing the HCO3 acetazolamide or/and ultrafiltration or hemodialysis by applying a low bicarbonate bath.
- By increasing PaCO2 by rebreathing in a closed system, or by controlled hypoventilation.
A simple method for mild hyperventilation due to anxiety is having the patient sit in a recumbent position, then putting one hand on the chest and the other on the abdomen. Advise the patient to change the breathing pattern in a manner to move the hand on the abdomen more than the chest, thus enhancing deep abdominal breathing. Patients who experience frequent hyperventilation should be evaluated for referral for psychotherapy, cognitive behavior therapy (CBT), or pharmacological therapy options.
Once stabilization is achieved, the underlying conditions and causative factors need to be treated.
Chronic respiratory alkalosis may require rebreathing in a closed system.

Self Quiz
Ask yourself...
- How would you categorize the clinical findings common with respiratory alkalosis?
- Does hypoventilation or hyperventilation correlate with this imbalance?
- Do you think it is important to determine the underlying cause?
- How would you explain the underlying pathophysiology of neurological symptoms such as confusion or disorientation with this imbalance?
Metabolic Alkalosis
Remember, the normal range for pH is 7.35 to 7.45, and an increase over this range is alkalosis.
Metabolic alkalosis is present when the pH is greater than 7.45. Primary abnormality is an elevated level of HCO3. Metabolic alkalosis is secondary to a metabolic process. (1)
It is important to recognize the physiological pH buffering process.
HCO3 functions as an alkalotic substance. CO2 functions as an acidic substance. Therefore, increases in HCO3 or decreases in CO2 will make blood more alkalotic; the opposite is also true that decreased HCO3 or increased CO2 will result in the blood being more acidic. (1)
CO2 levels are physiologically regulated by the pulmonary system through respiration, whereas the HCO3 levels are regulated through the renal system with reabsorption rates.
Causes of Metabolic Alkalosis
Many disease states can induce metabolic alkalosis.
The causes can be categorized as (3):
- Intracellular shift of hydrogen ions
- Loss of hydrogen ions through the gastrointestinal (GI) system
- Loss of hydrogen ions through the renal system
- Retention or addition of bicarbonate ions
Metabolic alkalosis will continue if the ability to eliminate bicarbonate is impaired due to one of the following causes: hypovolemia, reduced effective arterial blood volume, chloride depletion, hypokalemia, reduced glomerular filtration rate, and/or hyperaldosteronism (5).
The most common causes of metabolic alkalosis are (3):
- Diuretic use
- Volume depletion and loss of gastric acid and chloride (due to recurrent vomiting or nasogastric suction)
Clinical Findings in Metabolic Alkalosis
Mild alkalemia is usually associated with clinical findings of the underlying disorder. For example, hypokalemia will have classic symptoms.
As metabolic alkalemia becomes more severe, protein binding of ionized calcium (Ca++) increases, causing hypocalcemia. Hypocalcemia can present with headache, lethargy, and neuromuscular excitability (3).
Alkalemia also lowers the threshold for anginal symptoms and arrhythmias.
Management of Metabolic Alkalosis
The treatment of the underlying cause is the target of treatment for metabolic alkalosis, especially in hypovolemia and hypokalemia.
IV 0.9% saline solution is recommended for chloride-responsive metabolic alkalosis (3).
Cases of severe metabolic alkalosis (pH > 7.6) can require more aggressive methods of correcting blood pH; hemofiltration or hemodialysis is an option (3).
Oral or IV Acetazolamide can increase HCO3− excretion, but may also accelerate urinary losses of K+ and phosphate (PO4−) in volume-overloaded patients with diuretic-induced metabolic alkalosis.

Self Quiz
Ask yourself...
- What are the most common causes of metabolic alkalosis?
- How do the signs and symptoms differ among respiratory and metabolic alkalosis?
- Can you consider a healthy and functional renal system compared to an impaired renal system in this imbalance?
- What lab levels would indicate severe metabolic alkalosis?
Respiratory Acidosis
Respiratory acidosis occurs in alveolar hypoventilation, and the lungs are unable to excrete enough CO2. This causes PaCO2 to build up. (4)
As we mentioned earlier, an excess of CO2 combines with water to form carbonic acid, causing acidosis.
This is a common occurrence in emphysema (2).
The kidneys activate its compensatory process (albeit slow, often 24 hours or more) by increasing the excretion of metabolic acids through urination, which increases blood bicarbonate (4).
Types of Respiratory Acidosis (2)
There are two forms of respiratory acidosis: acute and chronic.
- Acute respiratory acidosis.
- Early stages
- Symptoms will progressively worsen.
- This can become life-threatening.
- Chronic respiratory acidosis
- Develops over time.
- There may be no symptoms
- The body adapts to the increased acidity.
Causes of Respiratory Acidosis
Respiratory acidosis is typically caused by an underlying disease or condition. This is also called respiratory failure or ventilatory failure.
- Hypoventilation
- Decreased ventilation leads to higher concentrations of carbon dioxide in the blood, which decreases the blood’s pH.
- Brain trauma, hypothyroidism: myxedema
- Chronic Obstructive Pulmonary Disease (COPD) and other Respiratory Conditions
- For those with COPD, the body attempts to compensate by retaining more bicarbonate.
- In respiratory conditions, the lungs cannot sufficiently eliminate carbon dioxide, which causes the pH to decrease.
- Overdose of an opiate or opioid.
Clinical Findings of Respiratory Acidosis
There are significant neurological signs and symptoms, including an altered level of consciousness, disorientation, drowsiness, headache, and confusion related to encephalopathy or cerebral edema. Tremors or shaking and jerking muscle movements may also be present.
Management of Respiratory Acidosis (2)
Respiratory acidosis management may include the following:
- Treatment of underlying conditions
- Bronchodilators and corticosteroids can be used to manage airway obstruction in conditions such as asthma and COPD.
- Mechanical ventilation through oxygen supplementation may be given.
- Management of hyperkalemia with Kayexalate.
- Acidosis causes potassium to shift from cells to extracellular fluid (plasma) in exchange for hydrogen ions, and alkalosis causes the reverse movement of potassium from extracellular fluid.
- Kayexalate increases potassium excretion.
- Administer intravenous fluids and electrolytes as ordered to maintain adequate hydration.


Self Quiz
Ask yourself...
- Can you describe common signs and symptoms associated with respiratory acidosis?
- How does hydration status impact the progression of this condition?
- What are the major goals of management?
- Can you think of conditions or pharmacological therapies that would lead to hypoventilation?
Metabolic Acidosis
Metabolic acidosis describes a decrease in bicarbonates and a buildup of lactic acid occurs.
Causes of Metabolic Acidosis
- Diabetic Ketoacidosis (DKA)
- DKA develops ketone bodies (which are acidic) build up during uncontrolled diabetes.
- DKA occurs mostly in Type 1 Diabetes Mellitus (DM).
- Chronic Renal Failure (CRF).
- Caused by tubular bicarbonate reabsorption and insufficient renal bicarbonate production.
- Chronic Hypoxia.
- It is related to lactate formation and pH falling to below 6.8.
- Obesity
- Diarrhea
- Causes loss of bicarbonate stores
- Characterized by increased plasma chloride and decreased plasma bicarbonate.
- Dehydration
- Electrolyte disturbances can cause metabolic acidosis.
- It is sometimes caused by prolonged vomiting.
- Aspirin Toxicity
- Aspirin overdose leads to an inability to produce ATP, leading to anaerobic metabolism.
- Aspirin overdose or poisoning can initially cause respiratory alkalosis, followed by metabolic acidosis.
- Methanol Poisoning. Significant methanol ingestion leads to metabolic acidosis, which is manifested by a low serum bicarbonate level. The anion gap is increased secondary to high lactate and ketone levels. This is probably due to formic acid accumulation.
Clinical Findings of Metabolic Acidosis
- Altered level of consciousness
- Confusion
- Disorientation
- Lack of appetite
- Coma
- Jaundice
Management of Metabolic Acidosis
Metabolic acidosis can be treated with the following:
- Treat the underlying condition.
- Sodium bicarbonate.
- Hydration is a major goal for diabetic ketoacidosis.
- Dialysis for chronic renal failure.
- The correction of metabolic acidosis mainly supplies bicarbonate during the dialysis sessions.
- Use of diuretics.
- Initiate safety measures.
- Kayexalate.
- Kayexalate binds potassium in the lumen of the gastrointestinal tract and increases fecal potassium excretion.

Self Quiz
Ask yourself...
- Can you describe common conditions associated with metabolic acidosis?
- How does the use of Kayexalate impact potassium levels?
- What are the major goals of management?
- Are you familiar with indications of lactic acid lab values?
Upcoming Research
Many clinicians insist that traditional arterial blood gas (ABG) analysis may overlook some metabolic acid-base disorders. Practice guidelines, such as Stewart’s approach in critically ill patients, suggest bicarbonate-anion gap-based methods (with and without correction for albumin) to diagnose acid-base those disorders (6).
Technology can improve the diagnosis and treatment of these imbalances, applying software tools for quicker and more efficient analysis of ABGs. Nurses should become aware of helpful tools and apps as they develop their practice in areas of intensive care and emergency medicine.
Let’s Practice!
ABG results:
pH = 7.56
Bicarb = 32
CO2 = 37
O2 = 90
Client complaint: Epigastric pain; note chronic use and overuse of antacids.
ABG results:
pH: 7.58
PaCO2: 20 mm Hg
PaO2: 197 mm Hg
HCO3 -: 20 mmol/L.
Client complaint: 15-year-old female complaining of tingling in all four limbs after being bullied by a classmate. This has occurred in the past and was diagnosed as peripheral neuropathy. The respiratory rate (RR) is 33/min.
Answer key:
- Metabolic Alkalosis. Is it compensated? No. The CO2 is within normal, and the pH is abnormal, showing uncompensated metabolic alkalosis.
- Respiratory Alkalosis. possibly due to fear, anxiety, or a panic attack.
Conclusion
Hopefully, you feel more confident in your detective skills when investigating arterial blood gas (ABG) analysis. This is crucial for nursing professionals to advocate for patients with a variety of respiratory and metabolic conditions. ABG analysis can seem intimidating, but once you memorize normal value ranges for ABGs, understand the pathophysiology of control of acid-base balance, and become skilled in recognizing which type of disturbance is occurring, your confidence will increase tenfold. Applying clinical findings and patient history ensures that nursing interventions are both precise and tailored to individual needs.
References + Disclaimer
- Brinkman JE, Sharma S. Physiology, Metabolic Alkalosis. [Updated 2023 Jul 17]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482291/
- Byrne, Deborah PhD, RN, CNE; Laske, Ann EdD, RN, CNE. Arterial blood gases: An easy guide to analysis. Nursing Made Incredibly Easy! 20(1):p 11-13, January/February 2022. | DOI: 10.1097/01.NME.0000801696.71591.cc
- Lewis, J.L. (2024). Metabolic Alkalosis. In: Merck Manual, Professional Version. Merck & Co., Inc., Rahway, NJ, US. https://www.merckmanuals.com/professional/endocrine-and-metabolic-disorders/acid-base-regulation-and-disorders/respiratory-acidosis#Key-Points_v8329400
- Mane, A. (2021). Arterial blood gas interpretation in clinical practice. Springer. https://doi.org/10.1007/978-3-030-69845-4
- Mane, A. (2021). Respiratory Alkalosis. In: arterial blood gas interpretation in clinical practice. In Clinical Practice. Springer, Cham. https://doi.org/10.1007/978-3-030-69845-4_4
- Paliwal, R., Pakavakis, A., Divatia, J. V., & Kulkarni, A. P. (2022). Utility of Stewart’s Approach to Diagnose Missed Complex Acid-Base Disorders as Compared to Bicarbonate-anion Gap-based Methodology in Critically Ill Patients: An Observational Study. Indian journal of critical care medicine: peer-reviewed, official publication of Indian Society of Critical Care Medicine, 26(1), 23–32. https://doi.org/10.5005/jp-journals-10071-24077
- Pruitt, B. (2024). Strategies for interpreting arterial blood gases. Nursing, 54(1), 16–21. https://doi.org/10.1097/01.NURSE.0000995560.71478.3f
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click the Green MARK COMPLETE Button Below
To receive your certificate
