Course
California APRN Course Bundle Part 1
Course Highlights
- Incorporate pharmacokinetic and pharmacodynamic principles of Schedule II controlled substances to safely prescribe medications in the part of care for patients experiencing pain.
- Analyze state laws, federal regulations, and evidence-based guidelines pertaining to furnishing, dispensing, and administering Schedule II controlled substances by a nurse practitioner.
- Identify components necessary for proper techniques of prescription writing for Schedule II through V consistent with the Health and Safety Code and Pharmacy law.
About
Contact Hours Awarded: , including 12 pharmacological contact hours.
Course By:
Multiple Authors
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Schedule II Controlled Substances and the Risks of Addiction
Introduction
Every clinician has cared for a patient in pain. Non-maleficence and beneficence, to do no harm and to do good, are the guiding ethical principles in patient care (16). Historically, easing pain and suffering was ethically straightforward- treating the pain was beneficial. Now, with the understanding of opioid misuse, clinicians may ease pain (beneficence), but cause opioid use disorder, abuse, and/or diversion and do more harm (maleficence).
Prescribing pain medications, especially schedule II controlled substances, comes with overwhelming responsibility and burden to the prescriber. The dual-edged sword of Schedule II controlled substances is to ease pain and to prevent misuse. To safely prescribe schedule II controlled substances, you must be aware of a myriad of facts, as well as clinically assess pain through the individual experience of each patient.
Case Scenario
Mary, a 34-year-old female patient you have cared for in the past for management of Type II diabetes, visits after moving furniture over the weekend. She is in apparent pain, holding her lower back and flinching with every movement. She is physically unkempt and appears exhausted. Mary states, “I’ve been trying to rest, I’ve iced the area and have been taking Ibuprofen every 6 hours and I can’t get any relief, I need something stronger.” What are your next steps?

Self Quiz
Ask yourself...
- How do you balance non-maleficence and beneficence regarding pain in your practice?
- How can you help prevent opioid-related use disorder, addiction, overdose, and/or deaths?
In 2016, the US CDC issued guidelines for the prescription of opioids to treat chronic, non-cancer pain. These more restrictive guidelines were partly in response to the growing number of people using opioids and the AHRQ 2014 findings. The guidelines were adopted by many states, including limiting prescriptions for opioids for the treatment of chronic, non-cancer pain. This event caused inadequate pain control and suffering for many patients who truly needed opioids but couldn’t obtain them through prescription because many states used the CDC recommendations as law.
In May 2021, California alone saw the sudden closure of twenty-nine pain management centers leaving more than twenty thousand opioid-prescribed patients without help or anywhere to go (13).

Self Quiz
Ask yourself...
- Have you been in a situation where you undertreated pain because of the law? If yes, how did you feel about this? If not, what would you do if you were in that situation?
Pain
Pain is a complex, not completely understood experience that is influenced by many components, including biological, psychological, and social factors (19).
Seeking relief from pain is one of the most common reasons patients reach out for medical care (19).
Pain Theory
Pain has existed since humans existed. The cause of pain has been explored for centuries. Even though our understanding of pain is still incomplete, there are many pain theories.
A brief, incomplete explanation of each theory is presented below (8,11):
- Intensity Theory: pain is an emotion.
- Cartesian Dualistic Theory: Pain is a consequence of committing immoral acts.
- Specificity Theory: Different sensations take different paths causing pain.
- Pattern Theory: Each sensation relays a particular pattern of signals to the brain, and then the brain reads the pattern to decipher the pain.
- Gate Control Theory: Pain travels from the periphery to the spinal cord. When the pain gets to a specific magnitude, the “gate” opens. After the spinal gate is open, the pain signal can reach the brain where it is processed, and lastly, the patient feels pain.
- Neuromatrix Model: The central nervous system is responsible for painful sensations, not the periphery. Pain messages to the areas of the central nervous system work together to create messages to allow patients to feel pain, called the neurosignature.
- Biopsychosocial Model: The biopsychosocial model is a comprehensive pain model encompassing all spheres of our humanness. This theory hypothesizes that pain is not made up of any one cause, but the result of multifarious psychological, biological, and sociological interactions. The theory links psychological and sociological interactions to the biological and helps us understand associated opioid use disorder, abuse, and diversion.
Case Scenario
Mary starts crying and says, “I know I deserve this pain; I had an abortion when I was in high school, and I guess I’m paying for it now.”

Self Quiz
Ask yourself...
- What pain theory drives your clinical practice? Why?
- How would you approach pain with a patient who believed in the Cartesian Dualistic pain theory?
- Do you judge or have a bias toward others based on the pain theory you ascribe to?
- How do you talk to Mary about her pain beliefs?
Case Scenario
After trying to explain that the pain was caused by the injury, not the abortion, Mary asks more about what actually causes the pain she is experiencing.

Self Quiz
Ask yourself...
- How do you explain the etiology of their pain to your patients in pain?
- Why is it important to share this information with the patient?
Types of Pain by Origin
There are different types of pain, depending on the origin. Determining the origin of pain is essential in the assessment and determining if treatment should involve Schedule II controlled substances. The most common causes of pain (acute and/or chronic) include (8):
Neuropathic Pain
Neuropathic pain can be peripheral or central, and it comes from nerve compression or nerve changes from other pathologies.
Peripheral neuropathic pain- examples include post-herpetic neuralgia and diabetic neuropathy.
Central neuropathic pain – examples include post-cerebral vascular accident.
Nociceptive Pain
Nociceptive pain is from direct tissue injuries, usually from an external force.
Examples include sprains, bruises, burns, or dental procedures.
Musculoskeletal Pain
Musculoskeletal pain originates from bones, joints, ligaments, tendons, or muscles.
Examples include arthritis, fractures, or back pain.
Inflammatory Pain
Inflammatory pain is due to the inflammatory response and associated swelling.
Examples include swelling from tissue injury, infection, or autoimmune disorders.
Psychogenic Pain
Psychogenic pain is caused by psychological factors.
Examples include tension headaches or stomach pain caused by stress.
Mechanical Pain
Mechanical pain is caused by pressure exerted on the body structure or part.
Examples include low back pain, abnormal growth, or tumors. (8)


Self Quiz
Ask yourself...
- Have you treated a patient with psychogenic pain? How did or would you validate their pain experience?
- What are non-pharmacological treatments you offer your patients depending on the pain’s origin?
Case Scenario
As you assess Mary and her pain, she tells you about the furniture moving last weekend, and that she fell while carrying a couch down a flight of stairs with another person. “I thought I was going to pass out! I had to stop right there and just cry from the pain. I had my friend take me home and this is the first time I’ve come out of the house since Saturday. I can’t sleep and I’m not hungry. My roommate is mad because I haven’t done a thing.”

Self Quiz
Ask yourself...
- What physical, emotional, and social repercussions is Mary experiencing due to her pain?
Pain Classifications
Determining the classification of pain- acute, chronic, or high-impact chronic will help the practitioner decide on appropriate treatment options.
Acute
Pain may be classified as acute pain. Acute pain comes on quickly and is limited (less than 1 month in duration) (18). Causes of acute pain can be a result of inflammation, injury, or a disease process. Acute pain interferes with daily functioning but usually subsides as the cause is treated. Acute pain may be described as throbbing, stabbing, or burning. Acute pain can cause physiologic symptoms including elevated heart rate and blood pressure.
Chronic
Pain can become classified as chronic when it lasts more than 3 months (8). Chronic pain is usually a result of injury, inflammation, treatment, or a pre-existing medical condition or disease (8). Every aspect of a patient's life may be affected by chronic pain leading to poor physical and mental health. When experiencing chronic pain, reduced quality of life, sleep, libido, and appetite changes are common (4).
High-impact Chronic Pain
In 2019, about 20% of adults in the US had chronic pain, and close to 7% had “high-impact” chronic pain, meaning they had pain every day or most days during the past 3 months that impacted normal work and life activities (5,6).
Assessing Pain
When contemplating prescribing opioids for chronic pain, thorough patient assessment including risk assessment of opioid use. Assessments of the patient’s pain encompass the origin, type, and intensity of the pain, past and present treatments, any underlying problems, and how pain affects physical and mental functioning (13). Patient-reported outcome (PRO) tools may simplify and organize the documentation of these goals and can be used to track patient progress over time (13).
As nurses, we all have been taught how to assess pain using the OPQRST mnemonic:
- Onset
- Provocation/Palliation
- Quality
- Region/Radiation
- Severity
- Time
And the 7 components of pain assessment:
- Onset/cause of pain
- Location/distribution
- Duration
- Pattern
- Character/quality
- Aggravating factors
- Alleviating factors/associated symptoms
These pain assessments only view the physical aspects of pain and are limited.
When assessing pain, these elements of pain experience need to be taken into consideration and include (11):
Nociception
The signal sent to the brain from the periphery that injury or damage is present. What is the origin of the pain?
Pain
The subjective experience after brain-processed nociception. What is the patient’s subjective pain experience?
Suffering
The emotional response to nociception. What is the patient’s emotional response to the pain?
Pain behaviors
The actions patients have in response to the experience of pain. What behaviors or changes does the patient have in response to pain?
Looking at these elements of the pain experience allows the practitioner to get a more holistic, individualized view of pain from the patient’s perspective.
Using reliable, validated tools to assess pain is needed to accurately measure and track pain levels. Mental health screening may be used to gather baseline information about and screen for any mental health concerns the clinician may have. Several Patient-Reported Outcome (PRO) tools are available and suggested pain screening tools (13):
- Pain Intensity and interference (pain scale)
- Brief Pain Inventory - Short Form (BPI-SF)
- PROMIS Pain Interference
- Mental health screening (the type depends on the practitioner’s evaluation and as appropriate)(13)

Self Quiz
Ask yourself...
- How differently do you view acute versus chronic pain and why?
- Do your personal experiences with pain affect how you perceive and address your patients’ pain?
- What are your pain biases physically, psychologically, and sociologically?
- What repercussions of pain have you experienced physically, emotionally, and socially?
- How were you taught to deal with pain as a child?
2022 CDC Guidelines for the Prescription of Opioids to Treat Chronic Pain
In 2022, the CDC issued new guidelines for prescribing opioids to treat chronic pain (5,6). The new guidelines support clinical judgment and individualized patient-centered care. Even though the CDC stresses they are recommendations, many states are again using the guidelines to make changes in state laws about prescribing opioids and other Schedule II controlled substances. Every prescriber must read the guidelines and review state prescribing laws from 2022 forward. The guidelines serve as a resource for prescribers, and the recommendations should be adhered to with the caveat to always individualize care and do the best for the given situation.
There are five guiding principles when implementing the recommendations into clinical practice. These broad guiding principles should be foremost when dealing with patients’ pain.
Five Guiding Principles of the Guidelines
- All pain, whether opioids are prescribed or not, needs to be assessed and treated on its own.
This reminds us that pain is pain and needs to be treated if opioids are prescribed or not.
- Flexible, supportive, individualized care should be voluntary, and all recommendations are supportive of person-centered care.
This reminds us that we are dealing with a unique person, and our care should reflect that fact and we need patient input for person-centered care.
- Pain should be managed using a multidisciplinary approach utilizing physical and behavioral health, and long-term services.
This reminds us we aren’t in it alone; a multidisciplinary approach allows the patient to receive the services they need from the appropriate provider.
- Make sure the clinical practice guidelines aren’t used beyond their intended use. Incorporate them with clinical judgment and patient-specific needs.
This reminds us that the guidelines are recommendations, and we need to use clinical judgment and the needs of the patient to drive our care.
- All layers of health systems need to be vigilant of health inequities and provide care and communication that is culturally and linguistically appropriate and accessible to all. Nonpharmacologic and pharmacologic pain management regimens should be affordable, diversified, and coordinated.
This reminds us that health inequities exist, and we need to provide care that is appropriate and accessible to all people to the best of our ability. (5,6)
Case Scenario
While explaining to Mary that you will give her printed care instructions before she leaves, Mary states, “Don’t bother, I can’t understand those instructions. They are way over my head, just tell me what I need to know.”

Self Quiz
Ask yourself...
- How do you stay vigilant of health inequities?
- What can you do to prevent health inequities in your practice?
- How will you help Mary with understanding her care?
Four Key Issues Addressed By the Guidelines
The four key issues addressed by the new guidelines include specifics on opioid prescribing (5,6). These guidelines may also be applied to prescriptions of other Schedule II controlled substances.
The first issue, whether to start opioids for pain should be addressed with every patient who is experiencing pain. The other three issues are after opioids are prescribed.
- Deciding whether to start opioids for pain.
Many factors need to be considered when deciding to prescribe opioids or not. Many times, pain can be controlled using nonpharmacological interventions and nonopioid medications.
- Choosing an opioid and the appropriate opioid dose.
There is no perfect way to decide on an initial opioid dose. In general, starting with a low dose is safer.
- Determining the length of time for the opioid prescription and conducting follow-up assessments.
Make sure to only prescribe the number of pills needed and schedule follow-up assessments.
- Assessing the risk for and educating on the potential harms of opioids.
Each patient taking opioids needs to understand the harms of opioids and be assessed for harm before, during, and after opioid therapy (6).
Case Scenario
As you explore treatment options with Mary, she tells you, “I told you I need something strong, the good stuff. The rest won’t do a thing for my pain!”

Self Quiz
Ask yourself...
- Do you think opioids are the best treatment for pain? Why or why not?
- Do your patients have the preconceived notion that opioids are “best” for pain control? How do you approach this notion?
Non-pharmacological Treatments for Pain
Nonpharmacological treatments for pain should be suggested as appropriate for patients in pain (6). These are usually cost-effective and have minimal downsides.
Examples of non-pharmacological treatments include (6):
- Exercise (aquatic, aerobic, and/or resistance)
- Application of heat/cold
- Elevation of affected body part
- Weight loss (for osteoarthritis or back pain)
- Massage
- Mindfulness-based stress reduction
- Yoga
- Acupuncture/acupressure
- Cognitive behavioral therapy
- Physical therapy
- Tai Chi
- Qigong
Case Scenario
While exploring non-pharmacological pain management options with Mary, she states, “My grandmother used to swear by hot baths with Epson salts for all pain! But why bother when you can take a pill, right?”

Self Quiz
Ask yourself...
- What non-pharmacological pain therapies have you seen patients use that are specific to their country of origin or passed down by generations?
- What non-pharmacological pain therapies have you used and why?
- How will you respond to Mary’s statement?
Non-schedule II Controlled Substances for Pain
As a prescriber of Schedule II controlled substances, remember that many non-opioid medications treat pain effectively (as or more effectively than opioids in many cases) including:
NSAIDS
Example: Ibuprofen
200 to 400 mg PO every 4 to 6 hours as needed. Max: 1,200 mg/day. Discontinue use if pain gets worse or lasts more than 10 days (15).
SNRI antidepressants
Example: Venlafaxine
37.5 mg PO once daily for 1 week, then 75 mg PO once daily for 1 week, and then 150 mg PO once daily. Doses up to 225 mg/day have been used. Guidelines state this medication is most likely effective and should be considered for the treatment of diabetic neuropathy (15).
Gabapentin
Example: Gabapentin
300 mg PO 3 times daily, at first. Titrate dosage is based on clinical response and tolerance. Max: 3,600 mg/day. Guidelines suggest gabapentin is most likely affective for diabetic neuropathy (15).
Case Scenario
You tell Maria that the ibuprofen she is taking is an excellent pain reliever and to continue taking it for the pain. She states, “I guess you haven’t been listening to me here, I still have pain so that means ibuprofen is useless to me.”

Self Quiz
Ask yourself...
- How do you use pain adjuncts in your practice?
- How do you respond when patients react as if you aren’t validating their pain when you prescribe non-opioids for pain relief?
- How do you explain to Mary why she should continue the ibuprofen?
Controlled Substance Act
Title II of the Controlled Substance Act (CSA) established federal regulation of controlled substances in 1970 (9). It was largely created to make a legal foundation to combat drug abuse.
This act also gave power to the Food and Drug Administration (FDA) and the Drug Enforcement Agency (DEA) to determine classification schedules. Mandatory registration through the US Attorney General controls and restricts who may import/export, manufacture, distribute, or dispense controlled substances.
Currently, there are five schedules of Controlled Substances (20). A brief description of each follows with emphasis on schedule II controlled substances.
Prescribers need to be aware of the specific, current information regarding each medication they prescribe and how that information relates to the individual patient receiving care.
Schedule I-V Controlled Substances
Schedule I Controlled Substances
These drugs have a high potential for abuse. Marijuana is the only Schedule I product that may be obtained legally in certain states in the US.
Examples (20):
- Heroin
- Lysergic acid diethylamide (LSD)
- Marijuana (cannabis)
- Peyote
- Methaqualone
- 3,4-methylenedioxymethamphetamine ("Ecstasy")
Case Study
Mary asks, “What about smoking pot for the pain? My neighbor told me I should and that it works great for his arthritis.”

Self Quiz
Ask yourself...
- How do you incorporate the discussion of legal cannabis for pain with your patients?
- How do you respond to Mary in this situation?
)Schedule II Controlled Substances & Schedule IIN Controlled Substances
Drugs in this schedule have a high potential for abuse that may lead to serious psychological or physical dependence. Oxycodone, hydrocodone, and hydromorphone tablets are all derived from poppy plants and are morphine derivatives whereas fentanyl is synthetic and much more potent than other Schedule II controlled substances. (15) Most opioids go through first-pass metabolism in the liver before entering the systemic circulation and reaching target tissues. There are individual differences in how opioids are metabolized because there are differences in a patient's CYP-450 and UGT liver enzymes that are part of the metabolizing process (15).
Examples of drugs in this class:
Morphine-opioid agonist
Morphine Tablets: 15 mg PO every 8 to 12 hours, at first. Titrate dose every 1 to 2 days to achieve adequate analgesia. While discontinuing, decrease the dose by 25% to 50% every 2 to 4 days to prevent withdrawal symptoms. Extended-release tablets are only prescribed for opioid-tolerant patients (15).
Hydromorphone-opioid agonist
Dilaudid: 2 to 4 mg PO every 4 to 6 hours PRN initially (15).
Fentanyl-opioid agonist
Duragesic: Follow the FDA-approved conversion chart to convert 24-hour oral morphine equivalents dose to the corresponding transdermal fentanyl system dose. To start, apply at minimum a 25 mcg/hour transdermal patch for patients receiving at least 60 mg/day oral morphine equivalents. All other opioids should be stopped with transdermal fentanyl initiation (15).
Methadone-opioid agonist
Dolophine: 0.05 to 0.1 mg/kg PO every 6 hours, to start. Titrate dose by 0.05 mg/kg/dose until symptoms are managed. Taper dosage 10% to 20% of initial dose every 1 to 2 days, lengthening interval before discontinuation (15).
Meperidine-opioid agonist
Demerol tablets: 50 to 150 mg PO every 3 to 4 hours PRN (15).
Oxycodone-opioid agonist
OxyContin: 5 to 15 mg PO every 4 to 6 hours PRN (15).
Hydrocodone-opioid agonist
Norco: 2.5 to 5 mg hydrocodone/325 to 650 mg acetaminophen (1 to 2 tablets) Q 4 to 6 PRN. Max: 30 mg hydrocodone/3,900 mg acetaminophen (12 tablets)/day (15).
Side Effects of Narcotics (Schedule II controlled substance)
Common side effects of narcotics (schedule II controlled substances) include:
- Nausea and vomiting (may also increase aspiration). Patients may need to be prescribed anti-emetics.
- Pruritus may cause skin irritation. Patients may need to take Benadryl to decrease itching.
- Dizziness- This is a safety concern, and the patient must know not to drive or be weary of falls.
- Dry Mouth- Hard candy or gum may alleviate this symptom.
- Sedation- This is a safety concern, and the patient must know not to drive or be weary of falls.
- Euphoria- Patients must understand not to drive, sign legal documents, or purchase items while “high.”
- Constipation-counsel to increase fluids and fiber in the diet, over-the-counter stool softeners may be recommended. (6, 20)


Self Quiz
Ask yourself...
- What are the most common side effects of opioids you see in your clinical practice, and how do you individualize interventions for your patients?
Schedule IIN stimulants examples:
- Amphetamine (Dexedrine, Adderall)
- Methamphetamine (Desoxyn)
- Methylphenidate (Ritalin)
- Amobarbital
- Glutethimide
- Pentobarbital (20)
Schedule III/IIIN Controlled Substances
These drugs have less potential for abuse than Schedule I or II drugs.
Examples:
- Medication with 90mg or less of codeine per dose
- Buprenorphine (Suboxone)
Examples of Schedule IIIN:
- Benzphetamine (Didrex)
- Phendimetrazine
- Ketamine
- Anabolic steroids (20)
Schedule IV Controlled Substances
These drugs have even less potential for abuse compared to Schedule III drugs.
Examples:
- Alprazolam (Xanax)
- Carisoprodol (Soma)
- Clonazepam (Klonopin)
- Clorazepate (Tranxene)
- Diazepam (Valium)
- Lorazepam (Ativan)
- Midazolam (Versed)
- Temazepam (Restoril)
- Triazolam (Halcion) (20)
Case Study
After reviewing Mary’s current medications, you see that she has been prescribed Triazolam by another provider.

Self Quiz
Ask yourself...
- How does having a patient on a schedule IV-controlled substance affect your decision whether or not to prescribe opioids for pain relief?
How Opioids Work
Opioids work by sending chemical signals that bind and activate opioid receptors. There are four known opioid receptors, and they include DOP, KOP, NOP, and MOP. A brief list of effects elicited by each receptor follows (10):
- DOP-spinal and supraspinal analgesia and decreased gastric mobility.
- KOP- spinal analgesia, diuresis, and dysphoria (like MOP without the vital sign changes)
- NOP- hyperalgesia, allodynia, and analgesia
- MOP-sedation, respiratory depression, analgesia, bradycardia, nausea, and vomiting, and decreased gastric mobility.
Opioids used in practice wield actions at the MOP receptor (James & Williams, 2020). The MOP receptor effects are the classic opioid effects that most clinicians see when caring for a patient taking opioids.
Opioids are highly addictive simply because they make you feel good. The release of endorphins triggered by opioids causes a sense of pleasure and well-being. As an opioid wears off, patients may find themselves craving the feel-good feeling again and take more opioids- not for pain, but to gain the good feeling back. There are psychological, genetic, and environmental factors that make patients at higher risk for abuse. They are also addictive because drug tolerance occurs and requires higher doses for the same effect.
The odds are a patient will still be on opioids a year after only five days on opioids (12).
Dosing
The first daily dose is a clinical decision made with the patient to provide individualized, appropriate care. When dosing opioids, use a Morphine Milligram Equivalent (MME) calculator. Use this tool to calculate the total daily opioid dose and document the MME. The total daily dose helps clinicians and patients figure out who may need additional monitoring, when tapering may be needed, and the risk of overdose (13). A patient's risks increase as the daily MME increases (13).

Self Quiz
Ask yourself...
- How do you use the MME as a clinical tool in your practice?
- What changes in your practice when you increase the MME for a patient?
Assessing the Effectiveness of Opioids
Suggestions for assessing the response to opioids for pain include:
Assessing the 4 A’s
- Analgesia- are they getting pain relief, what are the pain scores?
- Adverse effects- what side effects are they experiencing from the drug?
- Activity- how has the drug affected their activities of daily living including work and sleep?
- Aberrancies- anything out of the ordinary (such as asking for a refill early, taking the drug other than prescribed)? (13)

Definitions Regarding Opioid Misuse
A bit about definitions. These terms about the consequences of opioid use are often used interchangeably and incorrectly (7). Note the differences in each and make sure the patients know them too.
Addiction
The continual need for a drug despite harmful repercussions.
Example: A patient buys opioids off the street illegally because they physiologically need the medication and will go to any lengths to receive it.
Pseudo-addiction
The persistent fear of pain, causing hypervigilance which may go away when the pain resolves.
Example: A patient will not go anywhere without their medications in hand because they are afraid of being without pain treatment and limit many activities due to the fear of having more pain.
Dependence
The body needs medication to function normally, and physiologic withdrawal symptoms occur without the medication.
Example: a patient becomes anxious and starts sweating an hour before a medication is due.
Tolerance
The body needs more of the medication to achieve the same response caused by the CNS adjusting to a medication over a period of time.
Example: a patient's pain rating was between 4 and 5 (out of 10) on an opioid and after a week of therapy the pain rating increases (between 6 and 8) on the same dose, and they come to their prescriber requesting more drugs to get the same effect.
Opioid Use Disorder (OUD)
Encompasses dependence and addiction specifically to opioid use. OUD is defined in the DSM-5 as a problematic pattern of opioid use leading to clinically significant impairment or distress, as manifested by at least two DSM-5 criteria occurring within a 12-month period. This disorder is on a continuum and can be measured as mild, moderate, or severe.
Example: A patient has been on oxycodone for 3 years for back pain and their MME is 80. They admit physical addiction and emotional dependence on opioids.
Drug Diversion
The unlawful use or distribution of a drug.
Example: A patient comes in with complaints of severe pain with the intention of getting opioids to sell for cash.
Drug Misuse
Taking the drug differently than prescribed.
Example: A patient was prescribed 20 Tylenol #3 tablets to take Q6 hours PRN and comes to the clinic asking for more drugs in 3 days because they took them more often than prescribed. (7)

Self Quiz
Ask yourself...
- How do you respond if/when a patient or a colleague uses one of these terms incorrectly?
- What are the effects of incorrectly labeling someone as addicted?
Case Scenario
You decide to prescribe short-term opioid therapy for acute pain to Mary. What are your next steps?

Self Quiz
Ask yourself...
- How do you decide what and how much to prescribe?
- What are the state requirements to prescribe Schedule II controlled substances in California?
- What does Mary need to know about her prescription?
Prescribing in California
When prescribing Schedule II controlled substances in California, you must be aware of applicable laws and guidelines.
The Medical Board of California published guidelines in 2023 for prescribing controlled substances for pain. The steps to prescribing Schedule II controlled substances in California and the associated laws are briefly as follows (13).
Steps for Prescribing
- Establish a diagnosis and medical necessity.
The diagnosis should support opioid use, and the assessments support the medical necessity of the medication.
- Explore non-controlled medication treatment.
Again, many pain situations are controlled using non-controlled medications.
- If using Schedule II controlled substances: Use a patient-specific protocol for Schedule II medications.
Patient-specific protocols are required by California state law. A treatment plan should include a plan to discontinue or taper opioids as appropriate. The patient-specific protocol required nurse practitioners to furnish Schedule ll controlled substances, as defined in Health and Safety Code 11055 and 11056 (27,28), in a protocol, contained in the standardized procedure or protocols that specifies which categories of patients may be furnished this class of drugs.
- Make a treatment plan.
Treatment goals and objectives should be created when initiating an opioid trial. The goal of pain treatment should include documented improvement in pain, functioning, and a decrease in disturbances caused by pain. The plan should include an exit strategy of when and how the opioids will be discontinued.
- Obtain consent.
By creating a formal agreement and obtaining consent for opioid use, prescribers include their patients and can document their participation and enter the discussion of the gravity of opioid use.
- Enter a pain agreement.
The pain agreement can be tailored to the individual patient. Pain agreements can include safe medication administration and storage, what to do if medications are lost or if the pain increases. It can also include monitoring and compliance issues such as urine drug testing and pill counting. By entering a formal agreement, the patient realizes their accountability and responsibility in their pain management. Pain agreements can also be used to document teaching related to opioid use.
- Counsel on overdose and OUD prevention.
The eleven criteria for OUD should be discussed with the patient prior to prescribing opioids. This item is key to safety education. Beyond that, signs, and symptoms of overdose, how to use naloxone, and when to call 911 or seek medical attention are lifesaving instructions that patients need to know. According to California law, AB 2760, Wood, Chapter 324, Statutes of 2018, naloxone prescription must be offered to a patient when the dosage ≥ 90 MMEs (23).
- Ongoing assessments (pain, risk for misuse, risk of OUD).
While on opioid therapy, documentation of ongoing assessments is necessary to support your treatment plan and may prompt changes to the plan as assessments change.
- Compliance monitoring.
There are many aspects to compliance monitoring.
CURES/PAR
The law also requires compliance with California’s Prescription Drug Monitoring Program (PDMP) called CURES, AB 528, Low, Chapter 677, Statutes of 2019. To comply with CURES, all California-licensed prescribers are required to register for access to CURES once they are issued a Federal DEA Controlled Substance Registration certificate.
The California Controlled Substance Utilization Review and Evaluation System (CURES) is a database of Schedule II, III, and IV controlled-substance prescriptions dispensed in California. This system allows prescribers to review a patient's controlled-substance history. CURES is maintained by the Department of Justice.
CURES consult including A Patient Activity Report (PAR) generated from CURES (California’s prescription monitoring program) must be reviewed on each patient within 24 hours prior to prescribing a controlled substance according to AB 528, Low, Chapter 677, Statutes of 2019.
CURES consultation is required at least every four months while receiving treatment.
Other compliance measures may include:
Drug testing
Urine drug testing can monitor if the patient is taking the opioids as described and detect if they are concurrently using substances that may make them at higher risk for OUD.
Pill counting
Having the patient bring their prescription to visits and verifying that the number of pills in the container correspond to the number that should be there based on the prescribing frequency is an active form of compliance monitoring and can be a part of a pain agreement or consent to treat.
Drug diversion
If a patient is found to be participating in drug diversion, depending on the nature of the diversion, appropriate reporting to the DEA or legal actions may be necessary.
- Tapering and Discontinuing Opioid Therapy
Tapering or discontinuing opioids should be a part of the patient-specific plan and planned accordingly. (13)
Case Study
Mary appears overwhelmed and anxious as you go through the prescribing steps. She asks you, “Are you doing all this because you think I’m a drug addict?"

Self Quiz
Ask yourself...
- How do you respond to Mary’s claim of bias against her?
- How do you explain that “all this” is required by law and consistent with good practices, not to accuse her of being an addict?
California Prescribing Laws
As mentioned, there are California laws specific to prescribing Schedule II controlled substances and NPs. It is paramount that NPs keep abreast of prescribing laws as they change rapidly. In addition to the laws already mentioned, a brief review of California NPs’ legal requirements to prescribe schedule II -V medication follows.
AB II96, (Chapter) 748 1/2004 amended Business and Professions Code Section 2836.1 (21) gives nurse practitioners authority to prescribe Schedule II through V drugs. To prescribe in California, NPs need:
- BRN certification.
- An action furnishing number registered with the United States DEA (14).
- A DEA number.
- Complete continuing education requirements decided by the California Board of Registered Nursing.
- Pads used to write prescriptions for controlled substances need to have a 12-character serial number and a corresponding barcode according to AB 149, Cooper, Chapter 4, Statutes of 2019.
- Electronic submission of a controlled substance prescription is required according to AB 2789, Statutes of 2018.

Case Scenario
Mary asks for a paper copy of her prescription because it’s easy for her to drop it off at the pharmacy in the store where she does her grocery shopping.

Self Quiz
Ask yourself...
- How do you explain to Mary that you can’t give her a paper prescription without eroding her thin trust in you?
Patient Communication and Education
When prescribing opioids, patient education, and an open discussion on the potential harms of opioids is paramount for patient safety. Providers must effectively communicate with every patient, without bias and with cultural competence. By effectively listening and assessing each patient’s pain experience, the practitioner can more effectively and safely treat pain.
Essential topics for dialogue and discussion with patients before and during opioid treatment for acute and chronic pain include many aspects. Communication topics and suggestions from the 2022 CDC guidelines include (6):
- Make a plan to discontinue when prescribing opioids.
- Let a patient know whom and how to contact and protocols to follow for uncontrolled pain, so it can be quickly reassessed and managed.
- Explain respiratory depression and opioid use disorder-how to avoid, how to recognize, and how to treat. Teach that taking opioids with benzodiazepines, sedatives, alcohol, or illicit drugs increases the chance of respiratory depression.
- Advise patients of side effects and how to treat dry mouth, nausea, constipation, vomiting, drowsiness, and confusion.
- Initiate an opioid tapering plan if opioids are prescribed more than a few days.
- Teach that medication should only be taken as needed, not as often as prescribed. Encourage non-pharmacological treatments.
- Remind the patient the medication is to make the pain tolerable, not to eliminate it, not to make you “feel good.”
- Remind patients not to drive or operate machinery when taking opioids.
- Talk to patients about safe medication handling, storage, and no sharing. Include how to dispose of medications safely and naloxone for an overdose.
- Explain workplace toxicology testing and its potential to check the amount of opioids they are taking. Discuss using state prescription drug monitoring program (PDMP) data to evaluate the patients’ risk for an overdose.
- Explain why opioid prescriptions should only contain the quantity needed for the anticipated period of severe pain.
- Explain why within a month of prescribing opioids, the patient should be re-evaluated and prescription changes (escalating, de-escalating, or discontinuing opioids) made accordingly. If continued, re-evaluating will need to be performed at least monthly.
- Let the patient know if they show signs of opioid use disorder or addiction, you will offer, or arrange evidence-based treatment. Educate the patient that stopping on their own can be deadly, and they need medical help.
- Remind the patient there are no reliable ways to predict which patient will benefit from opioid prescriptions and which will be harmed (6).
Using these recommendations in your practice is essential for safely prescribing opioids. Review the complete CDC Guidelines for further information (6).

Self Quiz
Ask yourself...
- How do the 2022 CDC recommendations contrast to your current practice for opioid prescriptions?
- How can the 2022 CDC recommendations decrease opioid use disorder?
- How will you incorporate the guidelines in Mary’s case?
Risk Factors for Opioid Misuse
As a prescriber of opioids, you are responsible for understanding and recognizing opioid misuse, diversion, and OUD.
There are risk factors associated with the misuse of opioids. These risk factors increase the likelihood of opioid misuse or taking the medication differently than intended.
Risk factors of opioid misuse include (12):
- Being poor
- Unemployed
- History of substance abuse
- Environment that is high risk for misuse
- Adventurous or dangerous behaviors
- History of any mental disorder
- Stressful life
- History of drug or alcohol rehabilitation
- Female gender (12)
When patients have identifiable risk factors, prescribers should share this information with their patients, so they understand they are at risk for misusing opioids.
Tools for Assessment of Opioid Misuse Behavior
There are reliable and valid tools to assess opioid misuse behaviors, and the Medical Board of California endorses the use of these tools to assess opioid misuse behavior (13):
- TAPS
- SOAPP-R
- CRAFFT for adolescents (13)

Self Quiz
Ask yourself...
- What changes in your care when you have a patient that is at high-risk for misuse?
- Do you trust your patients that have opioid misuse behaviors and how does that impact your care?
Diversion
Drug diversion, or the illegal use or distribution of a drug, may lead to accidental overdose and a myriad of illegal activities (7). Prescribers need to be careful not to fall victim to drug diversion by protecting their prescribing information and watching out for patients who visit solely to receive narcotics.
Common Drug Diversion Activities include:
- Doctor shopping
- Prescription pad theft
- Selling drugs for money
- Giving drugs to someone other than to whom they are prescribed. (7)

Self Quiz
Ask yourself...
- What would you do if you found out your patient was selling their medication (Schedule II controlled substances) for money?
- Have you experienced a patient who visited you only to try to get narcotics (doctor shopping)? How did or would you respond in that situation?
Opioid Use Disorder
The term Opioid Use Disorder (OUD) encompasses opioid dependence and addiction and can be mild to severe. Opioid use disorder (OUD) currently affects over three million people in the United States (1).
The diagnosis of OUD is made by meeting at least two DSM-5 criteria of the eleven within a year, according to the DSM-5 (1). The key 11 criteria are as follows:
- Increasing dose/tolerance
- Wishing to cut down
- Excessive time spent getting or using the medication
- Strong want to use
- Use interferes with normal daily obligations
- Continued use despite life disruption
- Use of opioids in hazardous situations like driving
- Reduced interest in important activities
- Continued use despite physical and/or psychological problems
- Need for more of the medication for the same effect
- Withdrawal symptoms occur when the dose is decreased
Estimates support that less than 20% of people in the United States with OUD are receiving effective available treatment (7). Screening for OUD is part of prescribing. If OUD is found, start treatment, or arrange for the patient to receive treatment and further care from a substance use disorder treatment specialist certified by SAMHSA. Practitioners should not terminate care with a patient because of OUD, as this event could represent patient abandonment and is unsafe for the patient (6).
Case Scenario
Mary returns to the clinic for a follow-up five days after she was prescribed opioids. She tells you, “I’m really happy you went through all that stuff when you prescribed those painkillers to me. On that list of 11 things that I was supposed to watch for, I am already experiencing some. I don’t want to do anything on these drugs. There is no way I can be on these and live a normal life on them. I also found myself not wanting to take the pills or wishing I was off them because they messed up my life so badly. I want off.”

Self Quiz
Ask yourself...
- How did being proactive and following good prescribing practices impact Mary?
- How to you respond to Mary in this situation?
Tapering and Discontinuing Opioid Therapy
Discontinuing opioids can be achieved rapidly, as in the case of someone that was prescribed a 3-day course of opioids for an acute injury and healing has reduced the pain so opioids aren’t warranted, or slowly through tapering. Tapering is the reduction of the daily opioid dose or daily MME. Tapering should be used as an exit strategy for opioids for patients who have been on long-term opioids or anyone who has withdrawal symptoms when trying to discontinue. Tapering about 10% per month or slower is usually better tolerated than rapid tapers, especially when patients have been taking opioids for a year or longer (6).
Reasons for tapering include:
- Implementing the planned opioid exit as part of the patient's treatment plan
- Pain resolution
- Pain not resolved, and a new treatment plan without opioids is introduced
- Patient is experiencing impairment of daily functioning
- Patient is showing signs of OUD, misuse, or diversion
- Patient experienced an overdose or event leading to hospitalization. (6)
Adjunct drugs can be co-prescribed to help withdrawal symptoms and make the taper more tolerable (6).
Examples of Adjunct Medications include (15):
- Clonidine - Alpha-2-Agnonist for sedative and antihypertension effects
- Hydroxyzine - Antihistamine for nausea, vomiting, anxiety, and itching
- Loperamide - Antidiarrheal, for diarrhea
Opioid Withdrawal symptom severity can be measured by the clinician using the Clinical Opioid Withdrawal Scale (COWS) or patients may self-report severity using the Subjective Opiate Withdrawal Scale (SOWS) (13). Treatments can be based on the severity of the symptoms (13).
If a patient has OUD, start treatment immediately. The use of Buprenorphine is appropriate and is within the NP’s prescribing privileges as a provider of Schedule II controlled substances (6, 13).
Buprenorphine is a schedule III-controlled substance, a partial opioid agonist with pain-relieving and addiction-relieving properties. It reduces pain, withdrawal symptoms, and craving (15).
Depending on the severity of withdrawal symptoms, the NP may also arrange for the patient to get treatment from a substance use disorder treatment specialist certified by SAMHSA (6).

Self Quiz
Ask yourself...
- How have you implemented care with patients with OUD?
- What resources do you have and use to support and guide you with caring for patients with OUD?
Conclusion
Prescribing controlled substances is cumbersome, loaded with paperwork and forms, and full of legal caveats and ethical considerations. It is that way on purpose. When discouraged about the process, please remember all the people who have died or have had their lives destroyed by opioids before these laws and guidelines existed. The steps are taken to allow medication to do what it is supposed to do and address opioid misuse before it becomes a problem.
Schedule II Controlled Substances and Neonatal Abstinence Syndrome
Introduction
The utilization of Schedule II controlled substances raises significant concerns regarding risks of addiction. In the context of women's health, Neonatal Abstinence Syndrome (NAS) resulting from maternal use of addictive substances, including Schedule II controlled substances, remains a critical focus in California (7).
This dual challenge necessitates a comprehensive understanding of the legal, medical, and ethical aspects surrounding the use of these substances. This course explores the specific risks associated with Schedule II controlled substances in California, focusing on addiction and its potential impact on women and neonates.
Understanding why Schedule II medications are used in women's health lays the foundation for in-depth exploration of their pharmacokinetics, dynamics, risks, legal considerations, and patient-specific protocols.
Overview of Schedule II Controlled Substances
Schedule II controlled substances are a class of medications with recognized medical benefits but also a high potential for dependence, misuse, and abuse (11). The term "Schedule II controlled Substances" refers to substances classified under the Controlled Substances Act due to their high potential for abuse and addiction.
Medications in this category include oxycodone, hydrocodone, hydromorphone, meperidine, methadone, morphine, dextroamphetamine, methamphetamine, methylphenidate, secobarbital, pentobarbital, and fentanyl, among others used for managing severe pain and complex health conditions.
Since the potential for addiction to Schedule II controlled substances is a significant concern, healthcare providers must align with federal and state regulations emphasizing safe use, patient education, proper dosage management, and vigilant assessment to prevent escalating addiction issues.
Oxycodone, hydrocodone, and hydromorphone tablets are all derived from poppy plants and are morphine derivatives, whereas fentanyl is synthetic and much more potent (13). Most opioids go through first-pass metabolism in the liver before entering the systemic circulation and reaching target tissues. There are individual differences in how opioids are metabolized because there are differences in patients' CYP-450 and UGT liver enzymes, which are part of the metabolizing process (13).
Examples of drugs in this class:
Morphine - opioid agonist
Example: Morphine Tablets 15 mg PO every 8 to 12 hours, at first. Titrate dose every 1 to 2 days to achieve adequate analgesia. While discontinuing, decrease the dose by 25% to 50% every 2 to 4 days to prevent withdrawal symptoms. Extended-release tablets are only prescribed for opioid-tolerant patients (13).
Hydromorphone - opioid agonist
Example: Dilaudid: Give 2 to 4 mg PO every 4 to 6 hours PRN initially (13).
Fentanyl - opioid agonist
Examples: Duragesic -follow the FDA-approved conversion chart to convert 24-hour oral morphine equivalents dose to the corresponding transdermal fentanyl system dose. To start, apply at minimum a 25 mcg/hour transdermal patch for patients receiving at least 60 mg/day oral morphine equivalents. All other opioids should be stopped with transdermal fentanyl initiation (13).
Methadone - opioid agonist
Example: Dolophine- 0.05 to 0.1 mg/kg PO every 6 hours to start. Titrate dose by 0.05 mg/kg/dose until symptoms are managed. Taper dosage 10% to 20% of initial dose every 1 to 2 days, lengthening interval before discontinuation (13).
Meperidine - opioid agonist
Example: Demerol tablets- 50 to 150 mg PO every 3 to 4 hours PRN (13).
Oxycodone - opioid agonist
Example: OxyContin- 5 to 15 mg PO every 4 to 6 hours PRN (13).
Hydrocodone-opioid agonist
Example: Norco- 2.5 to 5 mg hydrocodone/325 to 650 mg acetaminophen (1 to 2 tablets) Q 4 to 6 PRN. Max: 30 mg hydrocodone/3,900 mg acetaminophen (12 tablets)/day (13).
Side Effects of Schedule II Narcotics (14, 15)
Common side effects of schedule II narcotics include:
- Nausea and vomiting may also increase aspiration. Patients may need to be prescribed anti-emetics.
- Pruritus may cause skin irritation. Patients may need to take Benadryl to decrease itching.
- Dizziness-this is a safety concern, and the patient must know not to drive or be weary of falls.
- Dry Mouth-hard candy or gum may alleviate this symptom.
- Sedation- This is a safety concern; the patient must know not to drive or be weary of falls.
- Euphoria patients must understand not to drive, sign legal documents, or purchase items while "high."
- Over-the-counter stool softeners may be recommended for constipation-counsel to increase fluids and fiber in the diet.
Schedule IIN stimulants examples (15):
- Amphetamine (Dexedrine, Adderall)
- Methamphetamine (Desoxyn)
- Methylphenidate (Ritalin)
- Amobarbital
- Glutethimide
- Pentobarbital
Schedule III/IIIN Controlled Substances (15)
These drugs have less potential for abuse than Schedule I or II drugs.
Examples:
- Medication with 90mg or less of codeine per dose
- Buprenorphine (Suboxone)
Examples of Schedule IIIN:
- Benzphetamine (Didrex)
- Phendimetrazine
- Ketamine
- Anabolic steroids
Significance of Using Schedule II Controlled Substances in Pharmacotherapeutic Management of Women's Health
Women's health conditions often demand a tailored approach due to physiological differences, hormonal fluctuations, and reproductive considerations. Pharmacotherapeutic management plays a crucial role in addressing the specific needs of women, and the significance of using Schedule II controlled substances cannot be understated.
These medications provide essential relief from pain and discomfort associated with various conditions often encountered in women's health, like endometriosis, postoperative pain, postpartum pain, fibromyalgia, or chronic pelvic pain, enabling women to lead healthier, more fulfilling lives. By closely monitoring patients, healthcare providers can harness the benefits of these medications while minimizing the risks (4).
Using Schedule II Controlled Substances Outside Acute Care Hospitals
While acute care hospitals are well-equipped to manage pain, many women's health conditions extend beyond these settings. As a result of that, utilizing Schedule II controlled substances outside acute care settings is a carefully considered approach in the pharmacotherapeutic management of women's health conditions. Let us see some reasons below:
Continuity of Care
Transitioning from inpatient to outpatient care requires maintaining a consistent level of pain management. For conditions requiring Schedule II controlled substances, continuation of therapy post-discharge ensures that pain relief remains adequate and uninterrupted.
Acute Postoperative Pain
Following surgical interventions, women may experience acute postoperative pain. Schedule II controlled substances can offer potent pain relief during immediate recovery, enabling patients to engage in necessary activities without excessive discomfort (2).
Reduction of Hospital Stay
In some instances, effective pain management with Schedule II controlled substances may lead to shorter hospital stays. Once stabilized, patients can be safely discharged with a prescription for continued pain relief, reducing healthcare costs and bed occupancy (2).

Self Quiz
Ask yourself...
- What does the term "Schedule II controlled substances" refer to?
- Are Schedule II controlled substances only needed in acute care hospitals?
Pharmacokinetics and Pharmacodynamics of Schedule II Controlled Substances
Pharmacokinetics and pharmacodynamics are essential pillars in pharmacology, offering insights into how medications interact with the human body. These principles play a pivotal role in Schedule II controlled substances, characterized by their potential for significant therapeutic benefits and inherent risks.
Understanding how these medications are absorbed, distributed, metabolized, and eliminated and how they exert their effects is paramount in ensuring safe and effective treatment for various health conditions. By understanding the details of pharmacokinetics and pharmacodynamics, healthcare providers can optimize treatment plans, mitigate risks, and improve patient outcomes (2).
Pharmacokinetics
Pharmacokinetics involves understanding how the body processes a medication from its administration to its elimination, and it involves four main stages: absorption, distribution, metabolism, and elimination.
Understanding the principles of pharmacokinetics is pivotal when considering Schedule II medications since the stages collectively determine a medication's effectiveness, duration of action, and potential interactions with the body. By grasping these concepts, healthcare providers can optimize treatment plans, ensuring safe and efficient outcomes for women's health conditions. Let us delve into absorption, distribution, metabolism, and elimination below.
Absorption
Absorption refers to the process by which a medication enters the bloodstream from its administration site, determining its onset of action and intensity of action. For example, after taking an oral dose of oxycodone, the medication is absorbed into the bloodstream through the stomach and small intestines.
For oral medications, the absorption rate of the specific medication and the presence of food in the stomach can influence the time it takes to be absorbed. On the other hand, Schedule II medications administered intravenously are rapidly absorbed, leading to swift pain relief as the medication directly enters the bloodstream.
Distribution
Distribution involves the transportation of the medication throughout the body, influenced by factors like blood flow, tissue permeability, and protein binding. In the case of patients with impaired blood flow, there might be a delay in the distribution of the medication, affecting the onset and duration of its effects.
Metabolism
Metabolism is the process by which the body transforms medications into metabolites, primarily occurring in the liver through enzymatic reactions. Patients with liver dysfunction might experience impaired metabolism, accumulating the medication and its metabolites, potentially resulting in adverse effects.
Elimination
Elimination involves the removal of the medication and its metabolites from the body, primarily through the kidneys via urine. Patients with impaired kidney function might face challenges in eliminating the medication, leading to its prolonged presence in the body and potential toxicity.
Pharmacodynamics
Pharmacodynamics explores how medications interact with the body to produce their effects. Understanding the mechanisms of action of Schedule II medications is essential for making informed treatment decisions. Let us explore these mechanisms below.
Receptor Interactions
Medications in this category work by binding to specific receptors on cells, triggering a cascade of events that lead to therapeutic effects (10).
These medications bind to opioid receptors in the brain and spinal cord, altering pain perception and relieving pain. A patient prescribed this opioid for pain management might experience varying degrees of pain relief based on individual differences in receptor sensitivity and medication affinity.
Enzyme Inhibition or Activation
Medications in this category influence enzymatic activity in the body by inhibiting or activating enzymes, impacting biochemical pathways (10).
These medications work either by inhibiting the reuptake of neurotransmitters to create therapeutic effects or by activating certain enzymes in the body to produce analgesic effects. Enzyme inhibition can lead to medication-medication interactions. Therefore, a patient on a Schedule II medication that inhibits a liver enzyme might experience altered metabolism of another medication, impacting its efficacy.
Cellular Signaling Pathways
Medications in this category modulate signaling pathways within cells, altering cellular responses and physiological processes (10).
These medications exert their effects by interacting with opioid receptors in the nervous system, leading to the modulation of signaling pathways involved in pain perception.
Ion Channel Modulation
Medications in this category impact ion channels, influencing the flow of ions across cell membranes and altering electrical activity (10).
These medications stabilize neuronal membranes and prevent seizures. Patients on medications that affect ion channels might need close monitoring, as changes in electrical activity can lead to adverse effects such as cardiac arrhythmias.
Transporter Interactions
Medications in this category influence membrane transporters, affecting the uptake or out-flowing of substances across cell membranes (10).
These medications enhance the reuptake of neurotransmitters, improving mood and emotional well-being. Transporter interactions can impact medication levels, so patients on medications that affect transporters might require dose adjustments when co-administering other medications that share the same transporter.

Application of Pharmacokinetic and Pharmacodynamic Principles to Women's Health Conditions
Applying pharmacokinetic and pharmacodynamic principles to women's health conditions involves considering the unique characteristics of women. Let us explore further below.
Pregnancy
Pregnancy alters blood volume, cardiac output, and metabolism, affecting medication absorption, distribution, and elimination.
For example, when a pregnant woman with chronic pain is prescribed a Schedule II medication, the dosage might need adjustments because her medication absorption might be enhanced due to increased blood flow. For this reason, a pregnant woman prescribed a Schedule II medication should have her treatment plan reassessed regularly to account for changing pharmacokinetics and potential fetal exposure.
Menstrual Cycle
Hormonal changes during the menstrual cycle can impact medication receptor sensitivity and responsiveness.
Therefore, a woman with endometriosis taking a Schedule II medication might experience changes in medication efficacy during her menstrual phase due to fluctuating hormone levels. As a result, the timing of medication administration for women with hormone-sensitive conditions can be optimized based on the menstrual cycle to align with peak receptor responsiveness.
Age
Aging reduces renal and hepatic function, affecting medication clearance and metabolism.
For example, due to decreased renal function, an older woman taking a Schedule II medication for pain management might experience delays in the medication's elimination might be delayed, necessitating lower doses. Regularly monitoring older women on Schedule II medications is crucial to prevent accumulation and potential adverse effects due to impaired medication elimination.
Hormonal Changes
Hormonal changes during menopause can influence medication receptor expression and sensitivity.
For example, hormonal shifts during menopause might alter the impact of medications on neurotransmitter receptors, affecting their efficacy. Therefore, medication selection and dosing adjustments for women experiencing menopausal hormonal changes should be guided by a thorough understanding of receptor interactions.
Metabolic Variability
Genetic variability can lead to differences in medication metabolism among women.
For example, in the case of two women with the same condition taking the same Schedule II medication, genetic variations might cause one woman to metabolize the medication more quickly, requiring higher doses for therapeutic effects. Genetic testing to identify metabolic variations can guide personalized dosing strategies for women on Schedule II medications.

Self Quiz
Ask yourself...
- What is the primary goal of understanding the pharmacokinetics of Schedule II controlled substances?
- How can understanding the Pharmacodynamics of Schedule II controlled substances aid in patient care?
Addiction and Neonatal Abstinence Syndrome
The risks of addiction associated with their use and the occurrence of Neonatal Abstinence Syndrome (NAS) in infants born to mothers who have used these substances. Understanding these risks is crucial for healthcare providers to provide informed care to women and their newborns.
Understanding Addiction
Addiction is a complex physiological and psychological phenomenon where an individual becomes dependent on a substance for various reasons, including pain relief or euphoria. Due to altered brain pathways associated with reward, addiction poses a significant risk, especially in patients with a history of substance abuse, genetic predisposition, or co-occurring mental health disorders (13).
Maternal addiction to Schedule II medications during pregnancy can lead to neonates having withdrawal symptoms. For example, a pregnant woman using Schedule II medications might unknowingly expose her baby to the substance, resulting in neonatal abstinence syndrome (NAS). As a result, women using these medications during pregnancy require specialized prenatal care to mitigate the risk of NAS and support neonatal well-being.
Identifying addiction signs and symptoms in pregnant patients is pivotal for timely intervention and ensuring maternal and fetal well-being. Healthcare providers need to be equipped to recognize the cues. Examples of such cues include behavioral changes like unexplained mood swings, irritability, anxiety, depression, frequent medical visits, discrepancies between self-reported medical history and prescription records, poor prenatal care adherence, unusual requests for specific medications, overusing or finishing prescriptions too quickly, social isolation, or poor self-care and nutrition.
By acknowledging addiction mechanisms, recognizing risk factors, and addressing psychological and physiological aspects, healthcare providers can implement preventive strategies, individualized treatment plans, and comprehensive care to ensure the well-being of women needing Schedule II medications.
Understanding Neonatal Abstinence Syndrome (NAS)
Neonatal Abstinence Syndrome (NAS) occurs when infants are exposed to medications, including opioids, in utero and experience withdrawal symptoms after birth.
Women who take Schedule II controlled substances during pregnancy can transfer these substances to their fetuses, resulting in a complex set of withdrawal symptoms experienced by the infants after birth, for example, irritability, tremors, feeding difficulties, vomiting, diarrhea, excessive crying, and even seizures. According to (9), it is essential to monitor and manage NAS to ensure the well-being of newborns and prevent long-term complications like developmental and cognitive challenges.
Tips
Healthcare providers must assess pregnant women for substance use, including prescribed medications, to anticipate the potential risk of NAS in neonates.
Long-term follow-up is essential to monitor the developmental progress of neonates with NAS and provide appropriate support as needed.
Preventive Strategies for Addiction and Neonatal Abstinence Syndrome
Preventive strategies for addiction and Neonatal Abstinence Syndrome are integral to promoting the well-being of pregnant patients and their newborns. By embracing preventive measures, healthcare practitioners contribute to reducing addiction risks and improving maternal and neonatal outcomes (7). Let us explore some preventive strategies below.
Patient Education
Empowered patients are better positioned to make informed choices, actively participate in their care, and contribute to addiction prevention and improved neonatal outcomes (12).
For example, suppose a nurse-midwife educates a pregnant patient about the potential effects of opioids on the developing fetus. In that case, the informed patient can be empowered to actively participate in treatment decisions while considering potential consequences and alternatives.
Healthcare providers can provide education on the following topics: The risks and benefits of Schedule II medications, how to recognize the signs of addiction, the importance of adhering to prescribed dosages, the potential risks of neonatal exposure to opioids and NAS, the importance of communicating openly with healthcare providers about substance use concerns, the importance of promptly reporting adverse effects of medication to allow for timely intervention and addiction prevention.
Screening and Assessment
Screening and assessment are pivotal in preventing addiction and Neonatal Abstinence Syndrome (NAS) associated with using Schedule II controlled substances. By identifying at-risk individuals, healthcare practitioners can tailor interventions, develop preventive measures, make informed treatment decisions, monitor progress, and provide timely support.
Effective screening and assessment benefits are significant and contribute to improved patient outcomes. For example, when a nurse-midwife incorporates substance use screening as part of routine prenatal assessments, there are higher chances of improved patient outcomes due to timely identification of risks and timely support.
Early Intervention Programs
Early intervention programs are crucial in preventing addiction and Neonatal Abstinence Syndrome (NAS) related to the use of Schedule II controlled substances. These programs provide proactive strategies to identify at-risk individuals, offer support, and implement preventive measures that improve patient and newborn outcomes (12).

Holistic Approach to Addressing Addiction and Neonatal Abstinence Syndrome (NAS)
A holistic approach to addressing addiction and neonatal outcomes involves recognizing the interconnectedness of physical, emotional, and social factors in maternal and fetal well-being. Healthcare practitioners, particularly nurse midwives, play a crucial role in adopting a comprehensive approach encompassing medical care, emotional support, and community resources. Let us explore the details below.
Comprehensive Assessment
Comprehensive assessment is a valuable tool in addressing addiction and Neonatal Abstinence Syndrome (NAS) linked to the use of Schedule II controlled substances (12). This holistic approach ensures that healthcare practitioners thoroughly evaluate the patient's medical history, substance use, and overall well-being. Comprehensive assessment leads to a holistic understanding of the patient's unique situation, resulting in personalized treatment plans.
The benefits of comprehensive assessment are significant, as they contribute to tailored interventions and improved outcomes. For example, when the nurse-midwife conducts a comprehensive assessment to understand a pregnant patient's addiction challenges and support needs, there is a high likelihood of promoting well-being.
Non-pharmacological Interventions
Non-pharmacological interventions offer valuable alternatives in addressing addiction and Neonatal Abstinence Syndrome (NAS) related to Schedule II controlled substances (12). These interventions focus on using alternatives other than prescription medications to manage pain, reduce reliance on opioids, and promote overall well-being.
Types of Non-Pharmacological Interventions for Women include physical therapy such as massage and acupuncture; emotional support, cognitive-behavioral therapies, mindfulness, meditation, and guided imagery; nutritional counseling focused on maintaining a balanced diet that supports overall health; exercise/movement; counseling and social support groups.
Non-pharmacological interventions for babies include swaddling, gentle rocking, and minimizing sensory stimulation to help soothe affected neonates.
Pharmacological Interventions
Pharmacological interventions offer valuable tools in addressing addiction and Neonatal Abstinence Syndrome (NAS) related to Schedule II controlled substances.
Types of Pharmacological Interventions include Opioid Substitution Therapy involving opioid agonists or partial agonists, such as methadone or buprenorphine, to mitigate withdrawal symptoms, support addiction treatment, and minimize neonatal exposure. Pain management medications such as non-opioid analgesics or local anesthetics can help control pain without opioids. These medications reduce the need for opioids, lowering the risk of addiction and NAS.
Pediatric doses of medications like morphine or methadone are used to gradually wean the neonate off the addictive substance, ease withdrawal, and ensure safe neonatal outcomes.
Multidisciplinary Collaboration
Multidisciplinary collaboration interventions are essential in addressing addiction and Neonatal Abstinence Syndrome (NAS) associated with using Schedule II controlled substances.
These interventions involve a coordinated effort among various healthcare professionals, including obstetrics, addiction medicine, neonatology, and mental health, to ensure comprehensive care for both the mother and the neonate. The benefits of multidisciplinary collaboration interventions include expertise from diverse specialties, tailored treatment plans, and a holistic approach to care.
Long-Term Recovery Planning
Long-term recovery planning is pivotal in addressing addiction and Neonatal Abstinence Syndrome (NAS) linked to Schedule II controlled substances.
Developing comprehensive recovery plans extending beyond pregnancy offers numerous benefits for individuals and their neonates (12). These benefits include sustained sobriety beyond pregnancy, reduced risks of relapse, reduced likelihood of NAS in subsequent pregnancies, and improved maternal health.

Self Quiz
Ask yourself...
- What is NAS?
- What should nurse-midwives consider when assessing the potential risks of addiction in women's health?
- What term describes the undesirable physiological effects experienced by infants born to mothers who used controlled substances during pregnancy?
- What should nurse-midwives monitor when a patient is undergoing treatment with Schedule II controlled substances?
- List two key components a nurse-midwife should consider when assessing the appropriateness of using Schedule II controlled substances for a patient.
Legal Framework for Dispensing Controlled Substances
Understanding the legal framework is crucial for healthcare providers to ensure compliance with state and federal regulations while providing safe and effective care. This module explores federal and state regulations, the distinctions between dispensing and administering, the nurse-midwife's role in adhering to legal requirements, and the implications for patient care.
Federal and State Regulations on Controlled Substances
Controlled substances, including Schedule II medications, are regulated by federal and state laws, resulting in the need for healthcare providers to be familiar with both sets of regulations. The Controlled Substances Act at the federal level categorizes medications based on their potential for abuse, and the Medication Enforcement Administration (DEA) is the government agency responsible for enforcing regulations.
State laws often complement federal regulations with additional requirements and guidelines. As a result, California's legal framework surrounding the use of Schedule II controlled substances underscores the need for careful adherence to state and federal laws and regulations (8).
Useful links
Code of Federal Regulations regarding controlled substances
California State regulations regarding controlled substances

Dispensing vs. Administering: Understanding the Difference
Dispensing involves the act of providing a patient with a prescribed medication. Administering, on the other hand, refers to physically giving the medication to the patient. Nurse-midwives have the authority to administer medications in various settings, but dispensing controlled substances requires a separate authorization or license, depending on state regulations (5). Understanding these differences is vital for healthcare practitioners to ensure compliance, patient safety, and effective medication management.
Key Differences
Role: Dispensing involves preparing and providing medications to patients for self-administration while administering entails healthcare professionals directly delivering the medication to the patient.
Location: Dispensing can occur at pharmacies or healthcare facilities, whereas administration occurs within healthcare or home settings.
Authorization: Dispensing requires a valid prescription, whereas administering necessitates an authorized practitioner's order.
Documentation: Dispensing involves proper labeling and instructions for patient use while administering mandates and accurate record-keeping of the medication's administration.
Control Measures: Dispensing adheres to controlled substance prescription requirements while administering requires adherence to patient-specific protocols and regulations.
Nurse-Midwife's Role in Ensuring Legal Compliance with Legal Requirements
Nurse-midwives are vital in ensuring legal compliance when dealing with controlled substances. This involves accurate record-keeping, understanding the scope of practice, and collaborating with other healthcare providers when necessary.
In addition to that, adhering to strict state laws and federal regulations is essential to safeguard patient safety, maintain professional integrity, and promote effective medication management. Staying updated on regulation changes and participating in continuing education are essential for maintaining compliance (3). Let us explore the nurse-midwife's responsibilities in legal compliance.
Understanding Legal Requirements
Nurse-midwives must comprehensively understand California's state laws and federal regulations governing Schedule II controlled substances because familiarity with their prescription requirements, documentation standards, and patient-specific protocols is crucial (8).
Prescription and Ordering
Nurse-midwives must ensure that prescriptions for Schedule II controlled substances are valid, accurate, and comply with legal specifications since properly authorized practitioner orders are essential for administering Schedule II medications (8).
Patient Assessment and Monitoring
Nurse-midwives are responsible for conducting thorough patient assessments to determine the appropriateness of Schedule II medications since ongoing monitoring of patients' responses to medications and their potential side effects is essential (1).
Patient Education and Informed Consent
Nurse-midwives must provide patients with comprehensive information about Schedule II medications' risks, benefits, and alternatives. Informed consent discussions should encompass potential addiction risks and neonatal outcomes, ensuring patients make well-informed decisions (1).
Documentation and Record-Keeping
Accurate and detailed documentation of medication administration, patient assessments, and communications is imperative because proper documentation ensures transparency, accountability, and compliance with legal requirements (6).
Collaborative Approach
Nurse-midwives must collaborate with healthcare teams, pharmacists, and other professionals to ensure coordinated patient care. Effective communication facilitates adherence to legal requirements and patient-specific protocols (1).
Continuous Education and Professional Development
Nurse-midwives must engage in ongoing education to stay updated with evolving regulations, guidelines, and best practices since professional development enhances the nurse-midwife's ability to navigate legal complexities and provide optimal care (1).

Self Quiz
Ask yourself...
- Which regulatory body oversees federal regulations regarding controlled substances?
- Which regulatory body oversees the state legal requirements for furnishing controlled substances?
- What license do nurse-midwives need to dispense Schedule II Controlled Substances?
- What does "dispensing" refer to in the context of controlled substances?
- Why is maintaining accurate documentation crucial in controlled substance management?
- Why do nurse-midwives need to analyze state laws and federal regulations regarding controlled substances?
- Why is it essential for nurse-midwives to stay informed about changes in controlled substance regulations?
Patient-Specific Protocols
Schedule II medications require a delicate balance between achieving therapeutic benefits and minimizing potential risks, including addiction and Neonatal Abstinence Syndrome (NAS). Patient-specific protocols play a pivotal role in ensuring safe and effective medication management. By adhering to these protocols, healthcare practitioners can ensure optimal patient care while navigating legal and ethical considerations.
Importance of Patient-Specific Protocols in Schedule II Controlled Substance Use
Patient-specific protocols are tailored guidelines designed to provide a clear and comprehensive approach to medication administration, considering the individual patient's needs, medical history, and unique circumstances like pregnancy status, concurrent medications, and potential contraindications. The importance of patient-specific protocols in administering Schedule II controlled substances includes the following:
They ensure that the administration of these medications is tailored to the patient's specific health status, ensuring that potential interactions, contraindications, and appropriate dosages are considered. This helps to minimize adverse events, side effects, unintended reactions, or complications associated with Schedule II medications.
They ensure that dosages are optimized based on age, weight, and metabolic rate. Customized dosages maximize therapeutic benefits while avoiding potential overdose risks.
They consider patient preferences, lifestyles, and cultural factors, promoting medication adherence. Improved adherence contributes to treatment effectiveness and better patient outcomes.
They address addiction risks associated with Schedule II substances by tailoring treatment plans and medication management to minimize misuse.
They ensure that healthcare providers comply with legal and ethical standards and promote bona fide patient-practitioner relationships when using Schedule II medications.
Components of Patient-Specific Protocols
Components of patient-specific protocols serve a specific purpose, grounded in patient safety, legal compliance, and effective medication management. Let us discuss the components below.
Scope and Purpose
This component clearly defines the scope and purpose of the protocol, specifying the medications covered and the conditions under which they will be administered, setting the context for healthcare practitioners.
Authorization and Qualifications
This component specifies the authorized healthcare providers who can execute the protocol, outlining the qualifications, training, and licensing required for practitioners to be eligible for administering Schedule II medications.
Patient Assessment
This component describes the required patient assessment criteria to determine Schedule II medication use appropriateness, highlighting factors such as medical history, current medications, and potential contraindications. This ensures patients receiving Schedule II medications are appropriate candidates based on medical history and current conditions.
Dosage and Administration
This component provides precise dosage guidelines for each medication covered in the protocol, specifying the route of administration, frequency, and maximum dosage limits to prevent adverse events.
Monitoring and Documentation
This component outlines the monitoring parameters that practitioners need to observe during and after medication administration, emphasizing the importance of documenting patient responses and any adverse effects.
Communication and Collaboration
This component highlights the importance of communication among healthcare team members when administering Schedule II medications and encourages collaboration to ensure comprehensive patient care.
Emergency Response
This component guides managing emergencies or adverse reactions related to Schedule II medications, giving detailed steps to be taken and resources to be utilized in case of unforeseen incidents.
Legal and Regulatory Compliance
This component addresses legal requirements, state regulations, and federal laws that must be followed when administering Schedule II medications while ensuring practitioners are well-informed about their responsibilities and liabilities.
Review and Update Process
This component specifies a process for regularly reviewing and updating the protocol to align with changing needs, medical practices, regulations, and guidelines.
Aligning Protocols with Business and Profession Code, Section 2746.51
Per the California Business and Professions Code, Section 2746.51, protocols for Schedule II controlled substance medications encompass several essential components. These components are meticulously designed to ensure patient safety, legal compliance, and effective medication management by nurse-midwives. Here is a list of the components:
Patient Assessment and Evaluation
This component involves evaluating the patient's medical history, current health status, existing medications, allergies, and potential contraindications (5). A comprehensive patient assessment is fundamental before furnishing a Schedule II controlled substance.
Medication Information
This involves giving detailed information about the specific Schedule II controlled substance, including the medication's name, dosage forms available, dosing guidelines, route of administration, and any relevant precautions (5).
Indications and Contraindications
This involves clearly outlining the conditions or situations for which the controlled substance is being prescribed (indications), as well as circumstances where its use is prohibited due to potential risks (contraindications) (5).
Dosage and Administration
This involves providing precise dosing instructions, including dosage strength, frequency, treatment duration, the appropriate administration method, and necessary preparation steps (5).
Monitoring and Assessment
This component specifies the parameters for monitoring the patient's response to the medication. It also involves outlining the desired therapeutic outcomes, potential side effects, and the plan for assessing the patient's progress (5).
Documentation and Record-Keeping
This involves emphasizing the importance of maintaining accurate and comprehensive documentation, including recording the patient's informed consent, the furnished medication details, the rationale for the prescription, and any follow-up assessments (5).
Emergency Situations and Adverse Reactions
This involves giving detailed steps to be taken in case of emergencies or adverse reactions related to the controlled substance, including the appropriate interventions and the procedure for seeking further medical assistance (5).
Collaboration and Referral
This component highlights the importance of interdisciplinary collaboration and communication, specifying when it is necessary to consult or refer the patient to other healthcare professionals based on the patient's response or changes in condition (5).

Self Quiz
Ask yourself...
- What is the significance of developing patient-specific protocols for controlled substances?
- Why must a patient's medical history be assessed before furnishing a controlled substance?
- What is the purpose of documenting patient assessments and medication prescriptions?
- Which regulatory body issues Furnishing Numbers to nurse-midwives?
- Explain the difference between "furnishing" and "administering" controlled substances.
- What is the purpose of outlining emergency procedures and adverse reaction management in patient-specific protocols?
- What is the primary responsibility of nurse-midwives when developing patient-specific protocols?
- Why is a bona fide patient-practitioner relationship meaningful in controlled substance management?
- What does the term "bona fide" mean in patient-practitioner relationships?
Conclusion
As we reach the end of this course, we celebrate your dedication to advancing your knowledge and expertise in women's health. In this course, we delved into essential principles, regulations, and considerations surrounding using Schedule II controlled substances in out-of-hospital patient care, including the pharmacokinetic and pharmacodynamic principles and the risks of addiction and neonatal abstinence syndrome associated with using Schedule II controlled substances.
With the insights and knowledge gained throughout this course, healthcare providers would be well-prepared to make meaningful contributions to women's health, advocate for patient safety, ensure ethical practices, and deliver patient-centered care.
Safe Prescribing of Opioid Drugs
Introduction
For centuries, humans have been using, developing, and synthesizing opioid compounds for pain relief. Opioids are essential for treating patients who are experiencing severe and sometimes even moderate pain. Chronic pain can negatively affect our lives. In 2011, the cost of chronic pain ranged from $560 to $635 billion in direct medical expenses, lost productivity, and disability. An estimated one in five U.S. adults had chronic pain (11).
Introduction of opioid drug class, indications for use, most prescribed opioids, and their effects.
Opioids usually bind to mu-opioid receptor sites, where they have agonist effects, providing pain relief, sedation, and sometimes feelings of euphoria. Opiates refer only to natural opioids derived from the poppy plant. The term "opioids" includes all-natural, semi-synthetic, and synthetic opioids.
Opioids are classified into several categories based on their origin and chemical structure:
- Natural Opioids (Opiates): These come from the opium poppy plant. Examples include morphine and codeine.
- Semi-Synthetic Opioids: These are natural opioids that are chemically modified. Examples include drugs like oxycodone, hydrocodone, oxymorphone, and hydromorphone.
- Synthetic Opioids: These opioids are entirely synthesized in a laboratory and do not have a natural source. Examples include fentanyl, tramadol, and methadone (4).
Providers must always caution patients about the benefits and risks. The main advantage of opioid medication is that it will reduce pain and improve physical function. A provider may use a 3-item Pain, Enjoyment of Life, and General Activity (PEG) Assessment Scale:
- What number best describes your pain on average in the past week?
- What number best describes how, during the past week, pain has interfered with your enjoyment of life?
- What number best describes how, during the past week, pain has interfered with your general activity?
The desired goal is a 30% improvement overall with opioid treatment. (Centers for Disease and Control)
Providers must also go over potential side effects and warnings. Side effects include sedation, dizziness, confusion, nausea, vomiting, constipation, itching, pupillary constriction, and respiratory depression. Providers should discuss the importance of taking medication as prescribed. Taking opioids in larger than prescribed dosage, or in addition to alcohol, other illicit substances, or prescription drugs, can lead to severe respiratory depression and death. Individuals should not drive when taking opioids due to the sedating effects and decreased reaction times.
There are now thousands of different Food and Drug Administration (FDA) approved opioids available for providers to prescribe. They can be administered via other routes and come in various potencies. The strength of morphine is the "gold standard" used when comparing opioids. A morphine milligram equivalent (MME) is the degree of µ-receptor agonist activity. The following is a sample of some of the most prescribed opioids and their MME:
| Opioid | Conversion factor | Opioid | Conversion factor |
| Codeine | 0.15 | Morphine | 1.0 |
| Fentanyl transdermal (in mcg/hr) | 2.4 | Oxycodone | 1.5 |
| Hydrocodone | 1.0 | Oxymorphone | 3.0 |
| Hydromorphone | 5.0 | Tapentadol† | 0.4 |
| Methadone | 4.7 | Tramadol§ | 0.2 |
To utilize this information, multiply the dose for each opioid by the conversion factor to determine the quantity in MMEs. For example, tablets containing hydrocodone 5 mg and acetaminophen 325 mg taken four times a day would include a total of 20 mg of hydrocodone daily, equivalent to 20 MME (11).

Self Quiz
Ask yourself...
- How often do you prescribe opioids to your patients? If so, which ones and what dosages?
- If you do not prescribe opioids, do you treat patients taking them?
- Have you ever felt hesitant about prescribing opioids? What were the reasons?
- What topics do you routinely cover when you discuss opioid prescriptions with your patients?
- Do you know how to determine if a patient is engaging in opioid-seeking behaviors?
A history of opioids leading to the current epidemic
While the current opioid epidemic has caused devastating effects in recent years, destruction from opioids has been going on for centuries. In the early 1800s, physicians and scientists became aware of the addictive qualities of opium. This finding encouraged research to develop safer ways to deliver opioids for pain relief and cough suppression, which led to the development of morphine. By the mid-1800s, with commercial production and the invention of the hypodermic needle, morphine became easier to administer.
During the Civil War (1861 to 1865), injured soldiers were sometimes treated with morphine, and some developed lifelong addictions after the war. Without other options for pain relief, physicians kept giving patients morphine as treatment. Early indicators that morphine should be used cautiously were largely ignored. Between 1870 and 1880, the use of morphine tripled. Even with the problems associated with opioids, they continued to serve a vital part in the pain treatment of patients, and their use and development continued (6).
Opioid prescribing increased fourfold during 1999–2010. Along with the increase in opioid prescriptions during this time, how they were prescribed also changed; opioids were increasingly prescribed at higher dosages and for longer durations. The number of people who reported using OxyContin for non-medical purposes increased from 400,000 in 1999 to 1.9 million in 2002 and to 2.8 million in 2003. This was accompanied by an approximately fourfold increase in overdose deaths involving prescription opioids (8).
In 2020, approximately 1.4 million people were diagnosed with opioid use disorder (OUD), of those associated with opioid painkillers, as opposed to 438,000 who have heroin-related OUD (1, 4). Over 100,000 people died of a drug overdose, with 85% involved in an opioid (1, 4).
Widespread efforts were made to combat this growing issue. The prescribing rate peaked and leveled off from 2010-2012 and has been declining since 2012. In 2021, an estimated 2.5 million adults had been diagnosed with OUD. However, the amount of MME of opioids prescribed per person is still around three times higher than in 1999 (5).
Controlled Substances
On July 1, 1973, the Drug Enforcement Administration (DEA) was established in the United States. The Diversion Control Division oversees pharmaceuticals. Within this Division are five levels of controlled substances, which classify illicit and medicinal drugs (7).
Schedule I Controlled Substances
No medical use, lack of accepted safety for use under medical supervision, and high abuse potential.
Examples of Schedule I substances are heroin, lysergic acid diethylamide (LSD), and marijuana (cannabis).
Schedule II/IIN Controlled Substances (2/2N)
High potential for abuse, which can lead to severe dependence.
Examples include hydromorphone, methadone, meperidine, oxycodone, and fentanyl. Other narcotics in this class include morphine, opium, codeine, and hydrocodone.
Some examples of Schedule IIN stimulants include amphetamine, methamphetamine, and methylphenidate.
Other substances include amobarbital, glutethimide, and pentobarbital.
Schedule III/IIIN Controlled Substances (3/3N)
Less potential for abuse than substances in Schedules I or II, and abuse may lead to moderate or low physical dependence or high psychological dependence.
Include drugs containing not more than 90 milligrams of codeine per dosage unit like Acetaminophen with Codeine and buprenorphine.
Schedule IN non-narcotics includes benzphetamine, phendimetrazine, ketamine, and anabolic steroids such as Depo®-Testosterone.
Schedule IV Controlled Substances
Have a low potential for abuse relative to substances in Schedule III.
Examples include alprazolam, carisoprodol, clonazepam, clorazepate, diazepam, lorazepam, midazolam, temazepam, and triazolam, Tramadol.
Schedule V Controlled Substances
Low potential for abuse relative to Schedule IV and primarily consist of medications that have small quantities of narcotics.
Examples include cough preparations containing not more than 200 milligrams of codeine per 100 milliliters or per 100 grams and ezogabine (10).

Self Quiz
Ask yourself...
- How have your prescribing practices of opioids changed in response to the epidemic over time?
- What trends have you noticed in overall inpatient treatment plans regarding opioid prescribing trends (e.g., dose changes? or increase in opioid alternative therapies?)
- Do you think there is a stigma surrounding patients who are currently using opioids?
Explore behaviors that indicate opioid seeking, misuse, or addiction in patients.
Opioid use disorder (OUD) causes significant impairment or distress. Diagnosis is based on the following criteria: unsuccessful efforts to reduce or control use or use that leads to social problems and a failure to fulfill obligations at work, school, or home. The term Opioid Use Disorder is the preferred term; "opioid abuse or dependence" or "opioid addiction" have negative connotations and should be avoided. (3).
OUD occurs after a person has developed tolerance and dependence, resulting in a physical challenge to stop opioid use and increasing the risk of withdrawal. Tolerance happens over time when a person experiences a reduced response to medication, requiring a larger amount to experience the same effect. Opioid dependence occurs when the body adjusts to regular opioid use. Unpleasant physical symptoms of withdrawal occur when medication is stopped. Symptoms of withdrawal include anxiety, insomnia, abdominal pain, vomiting, diarrhea, etc. (5).
Patients who have OUD may or may not have practiced drug misuse. Drug misuse, the preferred term for "substance abuse," is the use of illegal drugs and the use of prescribed drugs other than as directed by a doctor, such as using more amounts, more often, or longer than recommended or using someone else's prescription (3).
Some indications that a patient may be starting to have unintended consequences with their opioid prescription may include the following symptoms: craving, wanting to take opioids in higher quantities or more frequently, difficulty controlling use, or work, social, or family issues. If providers suspect OUD, they should discuss their concerns with their patients nonjudgmentally and allow the patient to disclose related concerns or issues. Providers should assess the presence of OUD using the DSM-5 criteria.
Providers can use validated screening tools such as:
- Urine and oral fluid toxicology testing
- Drug Abuse Screening Test (DAST)
- Tobacco, Alcohol, and/or other Substance use Tools (TAPS)
- A three-question version of the Alcohol Use Disorders Identification Test (AUDIT-C)
The following patients are at higher risk for OUD or overdose:
- History of depression or other mental health conditions
- History of a substance use disorder
- History of overdose
- Taking 50 or greater MME/day or taking other central nervous system depressants with opioids

Self Quiz
Ask yourself…
- How often do you have patients exhibiting symptoms of OUD in your practice setting?
- What would your next steps be if you identify a patient with potential OUD (e.g., additional screening, referral, or treatment plans)?
- Have you noticed any trends in patients presenting with OUD? Such as socioeconomic status, occupation, gender, race, and medical diagnosis.
The 12 components of the CDC’s recent guidelines for opioid prescribing.
In 2022, the CDC updated the 2016 guidelines to help prescribers navigate prescribing opioids amid an epidemic. These guidelines are directed toward prescribing medications to adults to be taken in the outpatient setting, for example, primary care clinics, surgery centers, urgent cares, and dental offices. These do not apply to providers caring for individuals with sickle-cell disease, cancer, those receiving inpatient care, or end-of-life or palliative care. They are also intended to serve as a guideline, and each treatment plan should be specific to the unique patient and circumstances.
Some of the Goals of the guidelines are to:
- Improve communication between providers and patients about treatment options and discuss the benefits and risks before initiating opioid therapy.
- Improve the effectiveness and safety of treatment to improve quality of life.
- Reduce risks associated with opioid treatment, including opioid use disorder (OUD), overdose, and death.
Recommendation #1: Determining when it is appropriate to initiate opioids for pain.
An essential part of the prescribing process is determining the anticipated pain severity and duration based on the patient’s diagnosis. Pain severity can be classified into three categories when measured using the standard 1-10 numeric scale. Pain scores 1-4 are considered mild, 5-6 are moderate, and 7-10 severe. Opioids are typically used for moderate to severe pain.
The patient’s diagnosis will allow the provider to determine if pain initially falls into one of the following three categories of anticipated duration: acute, subacute, and chronic. Acute pain is expected to last for one month or less. Acute pain often is caused by injury, trauma, or medical treatments such as surgery. Unresolved acute pain may develop into subacute pain if not resolved in 1 month. If pain exceeds three months, it is classified as chronic. Pain persisting longer than three months is chronic. It can result from underlying medical conditions, injury, medical treatment, inflammation, or unknown cause.
The CDC guidelines state that non-pharmacologic and non-invasive methods are the preferred first-line method of analgesia, such as heat/cold therapy, physical therapy, massage, rest, or exercises, etc. Despite evidence supporting their use, these therapies are only sometimes covered by insurance, and access and cost can be barriers, particularly for uninsured persons who have limited resources, no reliable transportation, or live in rural areas where treatments are not available.
When this is insufficient, non-opioid medications, such as Gabapentin, acetaminophen, or nonsteroidal anti-inflammatory drugs (NSAID), should be considered next. Selective antidepressants and anticonvulsant medications may also be effective. Some examples of when these drugs may be appropriate include neuropathic pain, lower back pain, musculoskeletal injuries (including minor pain related to fractures, sprains, strains, tendonitis, and bursitis), dental pain, postoperative pain, and kidney stone pain.
Providers, however, must also consider the risks and benefits of long-term NSAID use because it may also negatively affect a patient’s gastrointestinal and cardiovascular system. Depending on the diagnosis, a patient may also require an invasive or surgical intervention to treat the underlying cause to alleviate pain.
If the patient has pain that does not sufficiently improve with these initial therapy regimens, at that point, opioids will be the next option to be considered.
It does not mean that patients should be required to sequentially “fail” nonpharmacologic and non-opioid pharmacologic therapy or use any specific treatment before proceeding to opioid therapy. Example: A patient for whom NSAIDs are contraindicated has recently sustained a rotator cuff injury and is experiencing moderate pain to the point at which it is disturbing their sleep, and it will be several weeks before they can have surgery.
Recommendation #2: Discuss with the patient realistic treatment goals for pain and overall function.
Ideally, goals include improving quality of life and function, including social, emotional, and physical dimensions. The provider should help guide these patients to realistic expectations based on their diagnosis. This may mean that the patient may anticipate reduced pain levels but not complete elimination of pain. The provider should discuss the expected or typical timeframe where they may need medications.
If medications are anticipated for acute or subacute pain, a discussion about the expected timeframe for pain should be highlighted in the debate. The patient may then better understand if their recovery is progressing. For chronic conditions, the conversation will focus on or may emphasize the overall risks of beginning long-term medication therapy. They may also advise patients, particularly those with irreversible impairment injuries, that they may experience reduced pain but will not regain function. A withdrawal plan will be discussed if opioid therapy is unsuccessful or the risk vs. benefit ratio is no longer balanced.
The second section of recommendations covers the selection of opioids and dosages.
Recommendation #3: Prescribe immediate-release (I.R.) opioids instead of extended-release and long-acting (ER/LA) opioids when starting opioid therapy.
Immediate-release opioids have faster-acting medication with a shorter duration of pain-relieving action. ER/LA opioids should only be used in patients who have received specific dosages of immediate-release opioids daily for at least one week. Providers should reserve ER/LA opioids for severe, continuous pain, for example, individuals with cancer. ER/LA opioids should not be for PRN use. The reason for this recommendation is to reduce the risk of overdose. A patient who does not feel adequate relief or relief fast enough from the ER/LA dose may be more inclined to take additional amounts sooner than recommended, leading to a potential overdose.
Recommendation #4: Prescribe the lowest effective dose.
Dosing strategies include prescribing low doses and increasing doses in small increments. Prescribe the lowest dose for opioid patients. Carefully consider risk vs. benefits when increasing amounts for individuals with subacute and chronic pain who have developed tolerance. Providers should continue to optimize non-opioid therapies while continuing opioid therapy. It may include recommendations for taking non-opioid medications in addition to opioids and non-pharmacologic methods.
Providers should use caution and increase the dosage by the smallest practical amount, especially before increasing the total opioid dosage to 50 or greater morphine milligram equivalent (MME) daily. Increases beyond 50 MME/day are less likely to provide additional pain relief benefits. The greater the dosage increases, the tendency for risk also increases. Some states require providers to implement clinical protocols at specific dosage levels.
Recommendation #5: Tapering opioids includes weighing the benefits and risks when changing the opioid dosage.
Providers should consider tapering to a reduced dosage or tapering and discontinuing therapy and discuss these approaches before initiating changes when:
- The patient requests dosage reduction or discontinuation,
- Pain improves and might indicate the resolution of an underlying cause,
- Therapy has not reduced pain or improved function,
- Treated with opioids for a long time (e.g., years), and the benefit-risk balance is not clear.
- Receiving higher opioid dosages without evidence of improvement.
- Side effects that diminish the quality of life or cause impairment.
- Opioid misuse
- The patient experiences an overdose or severe event.
- Receiving medications or having a condition that may increase the risk of an adverse event.
Opioid therapy should not be discontinued abruptly unless there is a threat of a severe event, and providers should not rapidly reduce opioid dosages from higher dosages.
Patient agreement and interest in tapering will be key components of successful tapers. Integrating behavioral and non-opioid treatment and interventions for comorbid mental health conditions before/during a taper can help manage pain, strengthen the therapeutic relationship between the provider and patient, and improve the likelihood of positive tapering outcomes. When dosages are reduced or discontinued, a taper slow enough to reduce symptoms and withdrawal should be used. Patients should receive education on possible withdrawal symptoms and when to contact the provider.
For those taking opioids for a shorter duration, a 10% decrease of the original dose per week or slower until close to 30% of the initial amount is reached, followed by a weekly reduction of roughly 10% of the remaining dose) is less likely to trigger withdrawal. Tapers of 10% per month or less are better tolerated than rapidly tapering off when patients have been taking opioids for a longer duration (e.g., for a year or longer). Significant opioid withdrawal symptoms can indicate the need to slow the taper rate further. Short-term medications might also help manage withdrawal symptoms. Providers should follow up frequently (at least monthly) with patients engaging in opioid tapering.
Close monitoring is required for patients who cannot taper and who continue on high doses or otherwise high-risk opioid regimens and should collaborate with patients to mitigate overdose risk. Some patients with unanticipated challenges to tapering may need to be evaluated for OUD.
The third section focuses on the duration of opioid therapy and routine patient follow-up.
Recommendation #6: Prescribing no greater quantity than needed for the expected duration of severe pain requiring opioids.
A few days or less is often enough when opioids are used for common causes of nonsurgical acute pain. Many states have passed legislation that limits initial opioid prescriptions for acute pain to less than seven days. Many insurers and pharmacies have enacted similar policies. Providers should avoid prescribing additional opioids to patients if pain continues longer than expected.
Providers should prescribe and advise opioid use only as needed rather than on a scheduled basis (e.g., one tablet every 4 hours). Limiting the duration of therapy can decrease the need to taper. However, tapering may need to be considered if patients take these medications around the clock for more than a few days.
Longer durations of therapy may be needed when the injury is expected to result in prolonged severe pain (e.g., greater than seven days for severe traumatic injuries). Patients should be evaluated at least every two weeks if they are receiving opioids for acute pain. Suppose opioids are continued for a month or longer. In that case, providers should address potentially reversible causes of chronic pain so that the length of therapy does not continue to extend.
Recommendation #7: Evaluate the benefits and harms of opioid therapy regularly.
The benefits and risks for Evaluating benefits and risks within 1-4 weeks of starting long-term opioid therapy for subacute and chronic pain should be evaluated within 1-4 weeks of initiating therapy and after dosage increases.
The evaluation should include patient perspectives on progress and challenges in moving toward treatment goals, including sustained improvement in pain and function. The Three-item Pain, Enjoyment of Life, and General Activity (PEG) assessment scale could be utilized to help determine patient progress.
Providers should also ask patients about common adverse effects, such as constipation and drowsiness, and assess for outcomes that might be early warning signs for more serious problems such as overdose or OUD.
Patient re-evaluation should occur after therapy begins (about two weeks) when ER/LA opioids are prescribed if the total daily opioid dosage is greater than or equal to 50 MME/day or if there is a concurrent benzodiazepine prescription. These individuals are at a higher risk for overdose. Follow-up for individuals starting or increasing the dosage of methadone is recommended every 2-3 days for the first week. Providers should reassess all patients receiving long-term opioid therapy at least every three months.
The last section of recommendations covers patients at risk for OUD and overdose.
Recommendation #8: Use strategies to mitigate risk by evaluating risk for opioid-related harms, discussing risk with patients, and incorporating risk reduction strategies into the treatment plan.
The patient’s habits (including alcohol and illicit drug use) and behavioral and mental health must be considered. Patients with a history of substance use disorders, depression, and/or mental health disorders have a higher risk of overdose and OUD. Even though the dangers of opioid therapy are higher with these patients, they may still require opioid treatment for pain management.
Psychological distress can interfere with the improvement of pain and/or function in patients experiencing chronic pain; using tools like the Generalized Anxiety Disorder (GAD)-7 and the Patient Health Questionnaire (PHQ-9 or PHQ-4) to assess for anxiety, post-traumatic stress disorder (PTSD), and depression might help providers improve overall pain treatment outcomes. They should also ensure that treatment for depression and other mental health conditions is effective, consulting with behavioral health specialists when needed.
Additionally, providers should:
- Educate on the risks of overdose when opioids are combined with other drugs or alcohol.
- Use caution when prescribing opioids for people with sleep-disordered breathing due to their increased risk for respiratory depression. The provider may ascertain if a patient is compliant with prescribed CPAP.
- Use caution and increased monitoring for patients with renal or hepatic insufficiency.
- Use caution and increased monitoring for patients aged 65 years or older.
- Offering naloxone when prescribing opioids, particularly to patients at increased risk for overdose.
If patients experience a nonfatal opioid overdose, providers should evaluate for OUD. Providers should reduce opioid dosage, discontinue opioids when indicated, continue monitoring, and support for patients prescribed or not prescribed opioids.
Recommendation #9: Reviewing prescription drug monitoring program (PDMP) data.
Providers should review PDMP data specifically for prescription opioids, benzodiazepines, and other controlled medications patients have received from additional prescribers to determine all the opioids the patient could potentially receive. Patients with multiple prescriptions and from various providers are at an increased risk for overdose or OUD. PDMP data should be reviewed before initial drugs for subacute or chronic pain and at least every three months during long-term opioid therapy.
Recommendation # 10: Considering the benefits and risks of [urine] toxicology testing.
Toxicology testing should be used to inform and improve patient care. Providers, practices, and health systems should minimize bias in testing and not test based on assumptions about different patients.
Recommendation # 11: Use caution when prescribing opioid pain medication and other medications concurrently.
Benzodiazepines and opioids can cause CNS depression and potentiate opioid-induced decreases in respiratory drive. Because other CNS depressants can potentiate respiratory depression associated with opioids, benefits vs. risks should be considered.
Recommendation # 12: Offering or arranging treatment for OUD if needed.
Includes referring a patient to a specific treatment center where behavior therapy and medications may be prescribed.

Self Quiz
Ask yourself...
- Do you plan to update your prescribing practices with these new guidelines?
- How often do you assess your patients who require subacute or chronic opioid treatment for their response to treatment?
- How often do you suggest tapering or adjusting opioid dosages?
- Do you routinely screen patients for OUD who are receiving chronic therapy?
- What do you tell patients who would significantly benefit from opioid therapy (e.g., post-operative patients) who are afraid to take them due to adverse side effects?
Prescription Drug Monitoring Programs (PDMP) and Electronic Prescribing.
Prescription Drug Monitoring Programs (PDMP) is a database that keeps track of controlled substance prescriptions. It helps to improve opioid prescribing, inform clinical practice, and protect at-risk patients. A pharmacist must enter controlled substances into the state PDMP when dispensing them. When the pharmacist enters this data, it may occur at various intervals, from one month, daily, or even "real-time." However, a PDMP is only helpful if providers check the system before prescribing (10).
Some states have implemented legislation that requires providers to check a state PDMP before prescribing certain controlled substances and in certain circumstances. Most current mandates require that all prescribers query PDMPs when prescribing any opioid. Some states require prescribers to query PDMPs every time a controlled substance is prescribed, while others require a query only for the initial prescription. Subsequent checks of PDMPs also vary from every time a drug is issued to specific intervals (e.g., every 90 days, twice a year, annually) should prescribing continue.
Some mandates have categorical requirements; e.g., a query must be made if the prescription is over a three-day or seven-day supply or if a certain prescribed level of MME is exceeded. Other states' mandates are based on subjective criteria, e.g., a prescriber's judgment of possible inappropriate use or the prescriber's discretion regarding whether to query the PDMP. Finally, some states mandate that only prescribers in opioid treatment programs, workers' compensation programs, or pain clinics must query PDMPs (10, 11).
In addition to PDMPs, there has been an increase in requirements for providers to utilize Electronic Prescribing for Controlled Substances (ECPS). Electronic prescribing programs for both providers and pharmacies must meet DEA requirements. The DEA's March 31, 2010, conditions were updated on July 27, 2023 (12).
On January 1, 2023, the Centers for Medicare and Medicaid Services (CMS) implemented additional requirements for controlled substances for recipients of Medicare Part D. There are over 51 million U.S. people enrolled in Medicare Part D (Center for Medicare Advocacy, 2023). In addition to state laws, these rules require that prescribers e-prescribe at least 70 percent of controlled substances for patients that have Medicare Part D. A waiver may be approved if the prescriber cannot conduct electronic prescribing due to circumstances beyond the provider's control (12).
Starting June 27, 2023, the 'Consolidated Appropriations Act of 2023' requires new or renewing Drug Enforcement Administration (DEA) registrants, to have at least one of the following:
- A total of eight hours of training from specific organizations on opioid or other substance use disorders
- Board certification in addiction medicine or addiction psychiatry from the American Board of Medical Specialties, American Board of Addiction Medicine, or the American Osteopathic Association
- Graduation within five years and status in good standing from medical, advanced practice nursing, or physician assistant school in the U.S. that included an opioid or other substance use disorder curriculum of at least eight hours (11, 12).
Providers must follow either state law or DEA/CMS regulations, whichever is more stringent. The following map indicates various rules in each state, which will continue to change when new legislation is enacted (11, 12).


Self Quiz
Ask yourself...
- What are the laws in your state about PDMP and E-Prescribing?
- What references can you refer to find out prescribing laws in your state?
- Is the use of PDMP a common practice in your workplace?
Important teaching points about opioid storage and disposal.
Safe Storage
Patients should understand that prescription opioids need to be stored securely (i.e., keeping them in a locked area). This is especially true if children, teens, and other visitors in the house may be aware of their presence. Teens and young adults are the biggest misusers of prescription pain medication. In 2018, over 695,000 youths ages 12–17 and 1.9 million young adults ages 18–25 reported misusing prescription pain medication in the past year. Young people may misuse prescription opioids for many reasons, including curiosity, peer pressure, and wanting to fit in. Another reason teens and young adults may decide to take prescription opioids is because they can be easier to get than other drugs. Studies show that 53% percent of people over 12 who obtained prescription pain medication for non-medical use received them from a friend or relative (13).
Safe Disposal
Patients should be advised on how to get rid of unused or expired medications. The best option is to immediately take them to a drug take-back site, location, or program. These sites or programs can be found online, or the pharmacist may have information. If it is not feasible for the patient to get rid of the drug using a take-back program, the patient should be advised to check if it is on the FDA flush list. If it is, the medication should be flushed down the toilet. Again, the list of drugs is available on the FDA website. If it is not on that list, it should be discarded in the trash at home.
Patients should follow these disposal instructions: Mix medicines (liquid or pills; do not crush tablets or capsules) with an unappealing substance such as dirt, cat litter, or used coffee grounds. Next, place the mixture in a container such as a sealed plastic bag; then throw away the container in your trash at home. Last, the patient should remove or permanently cover all personal information on the prescription label of empty medicine bottles or packaging, then trash or recycle the open container (14).

Self Quiz
Ask yourself...
- Do you often teach your patients about the safe storage and disposal of opioids? If not, what are some barriers to providing this education? How could you overcome these barriers?
- Your patient asks if they can give the leftover pills to their spouse, who has back pain; what would be an appropriate response?
- The patient replies that it is the same medication their spouse has been prescribed and does not understand why they cannot share it; what education will you provide?
OUD treatment, including medications.
Treatment for OUD is multi-faceted and typically includes both mental health components and FDA-approved OUD treatment medications. Mental health components may consist of counseling or a structured treatment program. Cognitive behavioral therapy (CBT) may also be beneficial. A potential barrier to OUD treatment, on the provider's and patient's behalf, is the perception that patients must engage in counseling to start or continue receiving OUD treatment medication. While the mental health components are essential, there may be barriers for patients to begin mental health treatment programs, which include expense, travel, and available openings within the programs. Medication therapy may be a helpful start for these patients (9).
FDA-approved medications indicated for the treatment of OUD include methadone, buprenorphine, and naltrexone. Suboxone is a combination drug composed of buprenorphine and naltrexone.
Medication has several advantages as part of the OUD treatment plan.
- Help the individual to remain safe and comfortable during detox.
- Reduce or eliminate cravings for opioids
- Minimize relapse since the individual is not experiencing uncomfortable withdrawal symptoms
- Allow the individual to focus on therapy without being distracted by withdrawal symptoms and cravings
- Increase safety in cases of overdose
Methadone
Methadone is a full agonist opioid and is a Schedule II controlled medication. Methadone can be prescribed purely for the treatment of pain, as well as for OUD. Methadone treatment for OUD can only be provided through a Substance Abuse and Mental Health Services (SAMHSA)-certified opioid treatment program. Patients taking methadone to treat OUD must receive the medication under the supervision of a clinician. After consistent compliance, patients may take methadone at home between program visits. The length of methadone treatment should be a minimum of 12 months. Methadone doses are often adjusted and readjusted. Methadone is slowly excreted, and there is overdose potential if not taken as prescribed (9).
Buprenorphine
Buprenorphine is a partial agonist opioid. Buprenorphine can be prescribed by any provider with a current, standard DEA registration as a Schedule III Controlled Substance. Like opioids, it produces effects such as euphoria or respiratory depression. With buprenorphine, however, these effects are weaker than those of full opioids such as heroin and methadone. It also has unique pharmacological properties that help lower the potential for misuse and diminish the effects of physical dependency opioids, such as withdrawal symptoms and cravings (9). Subutex was a brand-name version of buprenorphine, discontinued in 2011 after new formulations that were less likely to be misused were developed.
Naltrexone
Naltrexone is an opioid antagonist, not addictive, and does not cause withdrawal symptoms. It blocks the euphoric and sedative effects of opioids by binding and blocking opioid receptors and reduces and suppresses opioid cravings. There is no potential for misuse and diversion. Naltrexone can be prescribed in any setting and can be taken as a pill or once monthly extended-release intramuscular injection (9).
It was estimated 2021 that of the 2.5 million people with OUD, only 36% received any treatment, and only 22% received medications. A part of the July 27, 2023, Consolidated Appropriations Act amended the Controlled Substances Act to eliminate the requirement that providers obtain a specific waiver (a DATA waiver) to prescribe buprenorphine (including Suboxone) to treat opioid use disorder, known as the X-waiver. Additionally, there are no longer any caps on the number of patients a practitioner can treat. This, however, does not change the requirements for methadone treatment (15).

Self Quiz
Ask yourself...
- What are some problems that can occur if opioid medications are not managing pain adequately?
- What are some possible ways you can obtain a detailed, patient-centric health history?
- What are some possible ways APRNs can educate patients on pain and opioid medication options?
Case Study #1
Patient Name: John Henderson,
Gender: Male
Age: 60
Height: 6' 1"
Weight: 190 lbs.
He is employed as a grocery store manager. Reports not using tobacco; drinks alcohol occasionally and has no illicit drug use. He has hypertension and high cholesterol and takes Losartan 50mg daily and Atorvastatin 20 mg daily.
This patient presents to a primary care office with pain and stiffness in the shoulder joint, which has progressively worsened for six months following rotator cuff surgery. He states his pain is unchanged, and he has a limited range of motion. It has been interfering with his ability to do his job. He said he went to physical therapy for a few weeks after his surgery but admitted he did not often complete the home exercise program regimen. An MRI was obtained and showed he had adhesive capsulitis. What are treatment options to consider for "frozen shoulder"?
Given the patient's diagnosis, non-opioid therapy options include nonsteroidal anti-inflammatory drugs, intraarticular glucocorticoid injections, steroid injections into the shoulder joint, range of motion exercises, physical therapy, and consulting with an orthopedic specialist who may recommend a joint manipulation under anesthesia. Each of these should be considered before opioid therapy.
Nursing Considerations
Nurses remain the most trusted profession for a reason, and advanced practice registered nurses (APRNs) are often pillars of patient care in several health care settings. Patients turn to nurses for guidance, education, and support. While there are no specific guidelines for the nurse’s role in opioid education and management, here are some suggestions to provide quality care for patients currently taking opioid medications.
Obtain a Detailed Health History
Often times, pain and mental health can be dismissed and overlooked in health care settings. If a patient is complaining of symptoms that could be related to pain, inquire more about that complaint. Ask about how long the symptoms have lasted, what treatments have been tried, if these symptoms interfere with their quality of life, and if anything alleviates any of these symptoms. If you feel like a patient's complaint is not being taken seriously by other health care professionals, advocate for that patient to the best of your abilities. A detailed pain assessment and history can provide context for opioid pain management and a patient's plan of care. When taking a health history, ask about any prior surgeries, major life stressors in the past year, or any prior opioid usage.
Review the Medication History
Often times, in busy clinical settings, reviewing health records can be overwhelming. Many people take pain medications, including opioids, for various reasons. Ask patients how they are feeling on the medication, if their symptoms are improving, if there are any changes to medication history, and if they use any other substances other than prescribed medications, such as alcohol, tobacco, or other drugs. Remember, prescription medications are not the only medications people take. Confirm medication route, dosage, frequency, and all the details to make sure you and the patient are on the same page and to avoid medication errors and complications. Medication history should be reviewed at every encounter.
Avoid Making Judgements
Society often stigmatizes open discussions of prescription medication and pain. Patients may avoid asking for pain medication for fear of being perceived as a "drug seeker." Other times, patients may have OUD and continuously ask for an increased number of opioid medications. Be willing to be honest with yourself about your comfort level discussing topics and providing education on opioid medications, drug interactions, and pain management. Be willing to address any questions/concerns the patients may have without making judgements.
Communicate the Plan of Care
Communicate the plan of care to other staff involved for continuity of care. For several patients, especially for patients with chronic pain or who use opioids long-term, care often involves a team of mental health professionals, physical therapists, nurses, specialists, pharmacies, and more. Ensure that patients' records are up to date for ease in record sharing and continuity of care and to reduce the incidence of opioid medication errors.
Engage in Self Learning
Stay up to date on continuing education related to opioid medications, pain management, and prescribing regulations. Evidence-based information and scope of practice is always evolving and changing. You can then present your new learning and findings to other health care professionals and educate your patients with the latest information. You can learn more about the latest research on pain management medications, non-pharmacological pain management options, and opioids by following updates from evidence-based organizations, such as the CDC or your local health department.
Perform Pain Assessments
As we know, it is not possible to look at someone with the naked eye and determine if they are in pain. Sometimes, it may be obvious when a patient is in pain (e.g., visible lacerations) and need pain management options, such as opioids. Other times, pain management is addressed as a result of taking a complete health history, listening to patient's concerns, completing a pain assessment, and offering testing to determine the cause of pain.
Assess for Opioid Use Disorder
While it is not possible to look at someone and determine if they have OUD, APRNs should pay attention to certain behaviors, for example, when a patient continually asks for more opioid medications or mentions that they are experiencing many symptoms common to those of OUD. OUD may be diagnosed as a result of completing a health history, listening to patient's concerns, and offering testing to determine the cause of pain. Remember, anyone can have OUD, and no two OUD patients appear the same.
Provide Patient Teaching
Patients should know that anyone has the possibility of experiencing side effects of opioid medications, just like with any medication. Patients should be aware that if they notice any changes in their breathing, changes in their heart rate, or feel like something is a concern, they should seek medical care. Because of social stigma associated with opioids and pain management, people may be hesitant to seek medical care because of fear, shame, and embarrassment. However, as more research and social movements discuss opioid use, there is more space and awareness for opioid education and opioid overdose prevention.
Nurses should also teach patients to advocate for their own health in order to avoid possible opioid complications and poor pain management.
Here are important tips for patient education in the inpatient or outpatient setting.
- Tell the health care provider of any existing medical conditions or concerns (need to identify risk factors)
- Tell the health care provider of any existing lifestyle concerns, such as alcohol use, other drug use, sleeping habits, diet, menstrual cycle changes (need to identify lifestyle factors that can influence opioid use and pain management)
- Tell the health care provider of any prior experiences with opioid medication (if applicable) and any medication reactions or side effects (need to identify risk factors, address pain management appropriately, identify any allergies, and avoid possible opioid overdose symptoms)
- Tell the health care provider if you have any changes in your breathing, bodily functions, or heart rate (potential opioid overdose symptoms)
- Tell the nurse or health care provider if you experience any pain that increasingly becomes more severe or interferes with your quality of life
- Keep track of your pain, overall health, medication use, and health concerns via an app, diary, or journal (self-monitoring for any changes)
- Tell the health care provider right away if you are having thoughts of hurting yourself or others (possible increased risk of suicidality and public safety concerns)
- Take all prescribed medications as indicated and ask questions about medications and possible other treatment options, such as non-pharmacological options or surgeries
- Tell the health care provider if you notice any changes while taking medications or other treatments to manage your pain (potential worsening or improving health situation)
Research
There is extensive publicly available literature on opioids medications. These can be found via the National Institutes of Health website and other evidence-based journals. As research is dependent upon the available of study participants, there are several ways people who take opioids can become part of research. If a patient is interested in participating in clinical trial research, APRNs can encourage them to seek more information on clinical trials from local universities and health care organizations.
Case Study #2
- Patient: Pilar
- Age: 40
- Height: 5' 1"
- Weight: 135 lbs.
Pilar presents to the urgent care clinic today complaining of a severe migraine, which started yesterday. Her history includes a hip injury following a car accident three years ago in which she developed chronic post-traumatic arthritis in the hip. After a total hip arthroplasty, she was diagnosed with heterotopic ossification (bone grows in tissue where it shouldn’t). For the past year, she has been taking 20 mg of oxycodone twice daily to manage chronic hip pain after unsuccessful non-opioid therapies. She has three children with no known pregnancy or postpartum complications. She previously worked part-time as an administrative assistant but has been off work since the car accident. She has post-traumatic stress disorder (PTSD) related to the accident. She has been prescribed Xanax 0.5mg up to three times daily for anxiety. She does not smoke, drink alcohol, or take illicit substances.
- What are some specific questions you'd want to ask about the hip arthritis?
- What are some health history questions you'd want to highlight?
- What lab work or testing would you suggest to perform?
For her migraine, Pilar stated she needed to take more of her oxycodone to deal with the pain and because she could not sleep last night. She is concerned because she is running out of pills. She said her primary care doctor's office was closed, so she came to the urgent care.
- What are some non-pharmacological interventions you can do for Pilar's pain?
- What are some questions you'd want to ask about her migraine?
- What are side effects of opioids would you discuss with her?
Here are some things to consider for Case Study #2.
- Discuss concerns with the patient. This includes taking the opioid more often than prescribed, potential problems with respiratory depression, and overdose.
- Recommend trying eletriptan or dihydroergotamine nasal spray first rather than additional opioids.
- Review the PDMP to see the prescription history for this patient. Attempt to contact the primary care provider to develop a plan of care.
- Consider conducting toxicology testing
- Consider offering naloxone
- Use the DSM-5 criteria to assess the presence (and severity) of OUD or arrange an assessment with a substance use disorder specialist. Offer treatment for OUD if it is confirmed.
Case Study #3
Sabrina is a 16-year-old Black high school student working as a waitress at a local restaurant. She arrives to the local pediatric emergency room after her shift with her mom because she thinks she is experiencing a sickle cell crisis. Sabrina reports that she has these crises every few months, and this is probably the third time she's been in this much pain. She reports being at this same ER last year for something similar. Her mother is completing paperwork and would like Sabrina to get some pain medication as well.
- What are some specific questions you'd want to ask about her health?
- What are some health history questions you'd want to highlight?
- What lab work or testing would you suggest to perform?
- What pain assessments would you perform on Sabrina?
Sabrina agrees to provide bloodwork, complete imaging, and be admitted. She said that no health care provider talked to her about how painful sickle cell crises can be, and she doesn't routinely take pain medication because she "doesn't want to be addicted." Sabrina and her mom heard about pain management options for these extremely painful episodes from social media and the internet and would like Sabrina to get her pain controlled. Sabrina said that she had some opioids last time she was in the ER, but she doesn't remember the name. Her mom doesn't remember the name either, but she remembers it was in an IV medication.
- How would you discuss Sabrina's pain management concerns?
- Given Sabrina's age, medical history, and prior history of opioid use, what medication options would be appropriate for a sickle cell crisis in an adolescent?
Sabrina has been in the pediatric ER for over a day receiving IV hydromorphone. She reports some relief, but Sabrina and her mom are concerned. Sabrina wants to live her life like a normal teenager without being in the hospital every few months for pain. Her mom asks if there is a way to have pain medication at home. Both Sabrina and her mom would like to know if there is anything that can be done to help with the pain outside of medications as well. Sabrina doesn't want to use pain medications daily but wants to have them at home just in case she can't get to the hospital.
- Knowing Sabrina's concerns, what are some possible non-pharmacological pain management options?
- Knowing Sabrina's health history, what would be some patient education talking points about at-home opioid medications and possible side effects?
- What are some possible consequences of leaving pain improperly managed?

Final Reflection Questions
- Are you familiar with any current research on opioid use?
- What are some reasons someone would want to enroll in clinical trials?
- How can nurses make a contribution to research?
- Do you plan to update your prescribing practices to reflect the new CDC guidelines?
Conclusion
Even providers who do not prescribe opioids should be familiar with the effects of opioids and OUD due to its high prevalence in the United States. Understanding the types of pain, how pain occurs, and how it impacts a person's quality of life is especially important. There is still an associated stigma among patients who use Opioids to treat chronic pain conditions. It is essential to recognize that there are times when opioid use is appropriate, as long as the provider practices the recommended guidelines and sound clinical judgment.
Tirzepatide for Type 2 Diabetes and Weight Management
Introduction
The emergence of the drug tirzepatide is becoming more popular and widespread and is being utilized among those with diabetes and also those who desire to lose weight. It is one of the newest diabetic drugs given by injection that also triggers dramatic weight loss in those who use the injections.
The U.S. Food and Drug Administration (FDA) approved tirzepatide in 2022 for individuals with diabetes, particularly Type 2 Diabetes. The FDA officials have not approved tirzepatide yet for weight loss, but they are currently tracking the medication and may have a recommendation for its approval by the end of this year. Clinical trials have shown that individuals with an elevated body mass index (BMI) and who did not have diabetes lost a considerable amount of weight when they received tirzepatide (1).
Advanced Practice Registered Nurses (APRNs) need to understand how to safely prescribe tirzepatide and the reasoning as to why it causes weight loss for specific individuals.
Drug Classification
Tirzepatide is part of a class of medications called glucose-dependent insulin tropic polypeptide (GIP) receptor and glucagon-like peptide-1 (GLP-1) receptor agonists. It comprises a 39 amino acid linear synthetic peptide conjugate to selective receptor agonists in preclinical and clinical trials.
Tirzepatide is used for treating Type II diabetes in adults as an adjunct to diet and exercise. It is also used for weight loss in some individuals and has gained increased attention as a new therapeutic agent for glycemic and weight control.
Social media has had a significant influence and increased the desire to use tirzepatide, and while individual results vary, the weight loss in adults ranged from 12 – 25 pounds.
Online pharmacies, diet clinics, and medical spas are implementing thousands of ads on social media to capitalize on a surge of interest in the drug.

Self Quiz
Ask yourself...
- Why has there seemed to be an increase in patients requesting this medication? What other medicines intended for type 2 diabetes are also being used for weight loss management?
- What are the ethical considerations regarding marketing this drug for weight loss when its primary use is for type 2 diabetes? Could this impact supply and costs?
Indications of Usage
The use of tirzepatide is being used for both Type II diabetes and weight control in certain patients. It has been a game changer for people living with Type II diabetes. The drug’s primary use is as an adjunct to diet and exercise to improve glycemic control in adults with diabetes.
The drug has also proven beneficial for weight loss in patients experiencing obesity, and those who are taking the highest dosage have shared a body weight reduction of 15.7% (2). Tirzepatide is an injectable prescription medication used together with diet and exercise, and it is not yet known if it can be used safely with patients who have had pancreatitis.
It is important to remember that it is not to be used for patients with Type I diabetes, but it is safe for Type II diabetic patients. Also, the safety of tirzepatide has yet to be discovered for children and those under 18; therefore, the medication should not be used for this age group.
In studies conducted with or without diabetic medicines, 75% – 90% of patients taking tirzepatide reached an overall A1C of less than 7% with an average starting A1C of 7.9 – 8.6% across the following dosages – 5mg, 10mg, and 15mg. The study results were measured at weeks 40 and 52 (3).

Self Quiz
Ask yourself...
- What dietary and activity recommendations can you provide to patients using tirzepatide for weight loss?
- Is this drug intended for those who want to lose 5-10 pounds?
Use of Tirzepatide with Diabetic Patients
Tirzepatide can be used for patients with Type II diabetes in combination with a diabetic-friendly diet and exercise. The drug works by lowering the patient’s overall blood sugar and also improves the A1C results of patients over some time. The injection has been approved by the FDA to treat Type II diabetes and is administered once weekly (4).
It is considered the first in a new class of medications – a dual glucose-dependent insulin tropic polypeptide (GIP) and glucagon-like-peptide-1 (GLP-1) receptor antagonist. The mechanism of how it works mimics two gut hormones (GIP and GLP-1). These hormones are essential in how patients digest food and regulate blood glucose after meals. The hormones also play a role in making individuals feel fuller and curb specific food cravings.
The provider can prescribe tirzepatide before attempting other diabetic medications if a patient has a BMI of 30 or greater or 27 or greater with weight-related conditions and if the drug is combined with a personalized weight loss plan that addresses physical activity, nutrition, and lifestyle changes.
However, due to the cost and some insurance companies not covering the injection unless the patient has both diabetes and obesity, the provider must carefully consider prescribing this medication.

Case Study
The patient states this ‘miracle drug’ is worth paying for out of pocket!
Jeff Capron, a 53-year-old Boonville, New York, web developer, started taking tirzepatide in December 2022. His friend had reported good results with the medication, so Jeff looked into the research studies behind it and then spoke with his primary physician.
The physician said, “Yeah, let’s give it a shot,” even though he did not have much experience with it. The physician did not have an opinion one way or the other than looking at the data set and seeing no reason why they could not try it.
Jeff’s hemoglobin A1C went from 10.1% to 6% in 3 months, which was very promising. “I never had that kind of experience with any medication for diabetes.” There is a range in how much A1C reduction people experience with tirzepatide, but many people taking it can get their A1C under 7% — an ideal goal for people with Type 2 diabetes.
Jeff experienced constipation and a little trouble sleeping early, but both issues disappeared quickly. He says, “I wake up in the morning, and my fasting blood sugars are normal.”
The medication took effect, he says, within 12 hours. He compared the feeling to having a gastric bypass.
“You cannot overeat food. As soon as you overeat, you almost feel ill.” While it generally takes a few months to notice effects like A1C reduction and significant weight loss, side effects such as lower appetite may be felt immediately.
Weight loss was not his primary goal, but he lost about 35 pounds on the medication in the first five months. He also lost his sweet tooth. “I can maybe count three sweet things I have eaten since December.”
Jeff found that his appetite slowly recovered days after taking tirzepatide. “You take the shot every Sunday, and by Saturday, you start to get a lot of appetite,” he says. “It does not seem to affect your weight. If I eat a little bit more on Saturday night, on Sunday, the scale will not move one way or the other.”
Jeff is allergic to hornets, so he already carries an auto-injector. He was not worried about using another drug delivered through a needle. “It’s just a push button,” he says. It also helped that his wife is a nurse. “So, I had her with me the first time to ensure I was doing it right. I didn’t even feel it.”
When Jeff was first prescribed tirzepatide, his insurance covered it. The company has since removed that benefit. He has filed an appeal but pays about $1,000 monthly out of pocket for his weekly injections. He plans to keep paying as long as necessary.
He considers the financial burden well worth it. “I have never had a medication that worked as well before for chronic conditions,” Jeff says. “I’ve been blown away by it. For me, it’s a miracle drug. It got rid of my diabetes” (4).

Self Quiz
Ask yourself…
- Can a provider willfully choose to prescribe tirzepatide before other diabetic medications are attempted?
- Would that impact his insurance coverage if Jeff did not meet the clinical criteria for using tirzepatide?
Use of Tirzepatide for Weight Loss Management
As mentioned, this medication is indicated for patients with a BMI of >30 or a BMI of >27 with qualifying comorbidities. Obesity can become a chronic lifetime disease, and for conditions such as these, the patient needs to implement therapy for the lifetime of the disease.
In a study conducted for tirzepatide, there was a dramatic increase in effectiveness compared to traditional nonsurgical interventions such as diet, exercise, and lifestyle changes. However, it has been noted that taking tirzepatide on an ongoing basis is recommended and necessary to maintain any weight loss achieved from the medication.
If a patient stops taking the drug, likely, it will no longer work (5).
Public health officials have expressed concerns about using the drug long-term. Still, data is currently lacking regarding long-term effectiveness, treatment duration, and maintaining weight reduction once the therapy is discontinued.
A recent trial consisted of 783 participants with a BMI greater than 30, and these participants agreed to take either a 10mg or 15mg dose of tirzepatide over 36 weeks. The injection is given once weekly, so this would equal a total of 36 injections.
By the end of 36 weeks, participants lost more than 21% of their body weight. After 36 weeks, participants continued on tirzepatide or received placebo treatment for the following year. The patients needed to be made aware of which treatment they were receiving.
Those still taking tirzepatide injections weekly after 88 weeks lost an additional 7% of their body weight, and those taking the placebo regained 15% at the end of 88 weeks (5).

Self Quiz
Ask yourself…
- What is the minimum BMI needed to qualify to receive this drug for weight loss management?
- Is this medication indicated for long-term use for patients with a high BMI?
Common Side Effects and Contraindications
Side Effects
Patients vary immensely with different experiences and side effects related to tirzepatide; however, the following are the most common side effects experienced by those taking the medication:
- Nausea
- Decreased appetite
- Vomiting
- Diarrhea
- Indigestion
- Constipation
- Stomach Pain
Tirzepatide usually does not cause fatigue, leaving one feeling weak, tired, and low energy. However, fatigue can be a common side effect of Type II diabetes.
It is important to note that most individuals who experience nausea, vomiting, and diarrhea episodes do so while the dosage increases, and typically, the symptoms decrease over time. G.I. effects were more prominent in those taking tirzepatide than those taking the placebo. The individuals not in the placebo group were more likely to stop treatment due to the unpleasant side effects (3).


Self Quiz
Ask yourself...
- Does tirzepatide cause fatigue in patients who use it?
Contraindications
Tirzepatide may cause thyroid tumors, including thyroid cancer, and it is essential to watch for possible symptoms, such as swelling or a lump in the neck, hoarseness, shortness of breath, or trouble swallowing.
Tirzepatide should also not be prescribed to any patient with Type 1 Diabetes.
One of the main ways that tirzepatide works is by stimulating the release of insulin from the pancreas, and due to this fact, there have not been many studies and clinical trials that include those with Type I diabetes.
However, this is not to say that prescribers have never ordered tirzepatide for those with Type I diabetes. Still, it is essential to note that if prescribed, it would be in addition to traditional insulin therapy.
- Personal or family history of a type of thyroid cancer known as medullary thyroid carcinoma (MTC).
- Any history of Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- Patients who are allergic to the actual medication or any of its ingredients.
- Younger than 18 years of age


Self Quiz
Ask yourself...
- What is the reason that tripeptide is contraindicated in those with Type I diabetes?
- Why is there a risk with patients who have a thyroid disorder?
Safe Prescribing Practices, Guidelines, and Considerations for Providers
Safe Prescribing Practices
As with all prescribed medications, safe standards of care must be implemented and followed to ensure patient safety is maintained. The same applies to providers considering prescribing tirzepatide, and specific criteria must be met beforehand. The following information discusses guidelines involving exclusion and inclusion criteria for providers to prescribe tirzepatide (6) accurately.
Guidelines
Exclusion Criteria – If present, the following indicates that the patient should not receive tirzepatide:
- Diagnosis of Type I diabetes
- Personal or family history of medullary thyroid carcinoma or with Multiple Endocrine Neoplasia syndrome type 2
- Severe gastrointestinal dysmotility
- History of pancreatitis
- Pregnancy
- Proliferative Diabetic Retinopathy (PDR), severe Nonproliferative Diabetic Retinopathy (NDR), clinically significant myalgic encephalomyelitis (M.E.), or diabetic macular edema (DME) unless the risks/benefits have been discussed with the patient and are documented in the patient's health record along with monitoring plans and follow-up with an eye specialist who is informed at the time of initiation.
Inclusion Criteria – All of the following must be met for tirzepatide to be prescribed:
- Diagnosis of Type II diabetes
- A BMI of 25 or greater
- Inadequate glycemic control on at least 1mg of semaglutide injection plus two or more glucose-lowering drugs
- Change needed to achieve goal A1C is less than 1%.
- Goal A1C should be based on those recommended in the Diabetic Guidelines.
- Adherence to current diabetic medications as evidenced by a review of the prescription refill history during the six months.
Additional Inclusion Criteria – All of the following must be met for tirzepatide to be prescribed:
- Patients with atherosclerotic cardiovascular disease or chronic kidney disease
- Patients of childbearing potential who are using oral contraceptives
Inclusion Criteria for Weight Loss
- BMI of >30 or >27 with patient weight conditions.

Self Quiz
Ask yourself...
- Would a patient with a BMI of 23 with no comorbidities qualify to use tirzepatide to lose 5-10% of their body weight? Why not?
- What impact can tirzepatide have on a person with a healthy weight and BMI of <25?
Considerations for Providers
There are specific considerations that prescribers must be aware of when contemplating if a patient should receive the medication tirzepatide. The following is imperative and must be considered each time the medication is prescribed to a patient:
- Clinical Indications – indicated for treating adults with insufficiently controlled diabetes mellitus as an add-on therapy to diet and exercise; as monotherapy when metformin is considered inappropriate due to contraindications or intolerance; and other medicinal products for treating Type II diabetes.
- Monitoring of medication – routine monitoring of serum calcitonin or thyroid ultrasound is of uncertain value but is recommended for early detection of Medullary Thyroid Cancer (MTC).
- Cost – the average price for tirzepatide ranges from $1,071-$1,351 without any coupons or insurance. Savings Card – manufacturer provided; patients can pay as little as $25 monthly for up to 12 injections. Savings Card – manufacturer provided; patients can pay as little as $25 monthly for up to 12 injections.
- Benefits and Risks – One must evaluate the effectiveness of diabetes and the weight loss experienced. Some of the risks must be evaluated, such as increased cost of medication, unpleasant gastrointestinal side effects, poor insurance coverage, and drug shortages. The FDA has warned that the medicine can cause thyroid C-cell tumors in rats, and it is not sure whether tirzepatide causes similar tumors.
How long does it take for tirzepatide to begin working?
Tirzepatide will start to lower one's blood sugar levels immediately, but it can take 8 to 12 weeks to reach one's target A1C goal.
Compared to other diabetic treatments, studies have shown that it can take eight weeks to reach an A1C target of less than or equal to 7% and 12 weeks to get an A1C of less than or equal to 6.5%. Significant weight loss can occur as early as 28 weeks.
Safe Administration
It is essential to follow the correct steps for safe administration of tirzepatide as listed below:
- The recommended starting dosage is 2.5mg, injected subcutaneously once weekly. The 2.5mg dosage is for treatment initiation and not for glycemic control.
- After four weeks, increase the dosage to 5mg, injected subcutaneously once weekly.
- If additional glycemic control is needed, increase the dosage in 2.5mg increments after at least four weeks on the current dose.
- The maximum dosage is 15mg, injected subcutaneously once weekly.
- If a dose is missed, instruct patients to administer it as soon as possible, within four days (96 hours) after the missed dose. If more than four days have passed, skip the missed dose, and administer the next dose on the regularly scheduled day. In each case, patients can then resume their regular once-weekly dosing schedule.
- The day of weekly Administration can be changed, if necessary, as long as the time between the two doses is at least three days (72 hours).
- Before initiation, train patients and caregivers on proper injection techniques.
- Instruct patients using the single-dose vial to use a syringe appropriate for dose administration (e.g., a 1ml syringe capable of measuring a 0.5 mL dose).
- Administer the medication once weekly, any time of day.
- Inject the medication subcutaneously in the abdomen, thigh, or upper arm.
- Rotate injection sites with each dose.
- Inspect the medication visually before use. It should appear clear and colorless to slightly yellow. Do not use the medicine if particulate matter or discoloration is seen.
- When using the medication with insulin, administer it as separate injections and never mix. It is acceptable to inject tirzepatide and insulin in the same body region, but the injections should not be adjacent.
Does the tirzepatide injection hurt when administered?
Pain from the injection site has not been reported as a common side effect, but it may occur.
Due to the injection being given subcutaneously, slight pain or discomfort can occur.

Self Quiz
Ask yourself...
- The patient asks you," How long will this take to work?" How will you respond?
- The patient reports they have never used an injection before; what methods can you use to teach your patient how to administer this medication safely?
Alternatives to Tirzepatide for Weight Loss Management
In some instances, patients need to be aware of alternatives to tirzepatide in case they cannot take the actual injection for whatever reason. In cases such as these, there are alternative supplements that can be purchased over the counter, and they include the following (7):
- PhenQ – top OTC choice – comprehensive weight loss solution that targets specific body regions, facilitates prompt fat loss, and expedites the weight loss journey.
- PhenGold – the most potent OTC weight loss alternative – one of the top weight loss supplements that boost metabolism, making one less hungry, less tired, and an overall improved feeling.
- Capsiplex BURN – the best choice for men – helps to burn fat faster and keep blood sugar levels in check. It helps to keep one's muscles, curbs hunger, gives one more energy, and torches stubborn fats.
- Trimtone – the best choice for women – helps women to lose weight, eat less, increase metabolism, burn extra calories, and boost energy.
- Prime Shred – best fat burner for men – boosts metabolism, keeps muscles intact, increases energy, and helps maintain focus.
The advanced practicing nurse or prescriber needs to inform patients about alternative options such as these in an effort for individuals to understand that other choices are available and can be used. Many individuals need to be more knowledgeable about alternatives besides tirzepatide due to the extra hype from social media sources that promote advertisements related to tirzepatide only but do not mention the other options.
Why does social media influence and encourage patients to take tirzepatide?
Social media trends can be helpful but can also become harmful by setting unrealistic expectations and promoting a diet culture mentality. They can create an unhealthy obsession with "clean" eating, especially in the younger populations.
Due to this, many individuals take the medication despite any occurrence or history of Type II diabetes, and the drug can ultimately become misused.
It has been noted that there is an influx of patients requesting this medication for weight loss instead of the intended purpose, which is to help control Type II diabetes.
Tirzepatide represents one of the most recent non-medical treatments aimed at managing the symptoms of Type II diabetes. While it is not indicated for weight management, diabetic patients who receive it frequently report a significant reduction in body weight.
Empirical evidence suggests the efficacy of tirzepatide in weight management, and certain physicians currently endorse the Administration of the medication as a therapeutic and effective means to overcome obesity.
What are some severe side effects of tirzepatide that can impact patient safety?
The Administration of tirzepatide can benefit many individuals, but some severe side effects must be mentioned.
These include thyroid tumors, thyroid cancer, pancreatitis, hypoglycemia, serious allergic reactions, kidney issues, severe stomach problems, vision changes, and gallbladder issues. All these side effects must be taken seriously and reported, as they can lead to life-threatening

Self Quiz
Ask yourself...
- With what you have learned in this course, what education will you provide to patients requesting this medication for weight loss?
- Have you seen increased demand for this medication in your current practice?
- If you Google tirzepatide, your results will likely include links to telehealth services promoting this weight-loss medication. To determine eligibility, what special considerations need to be taken to assess a telehealth patient?
Conclusion
Medications like tirzepatide are game changers for those patients with type 2 diabetes that have failed other medications. Unfortunately, several companies seek to profit from its weight-loss benefits through aggressive marketing campaigns that limit the available supply and increase the costs for those who need it. As healthcare providers, we need to use sound clinical judgment and follow the exclusion/inclusion criteria and other guidelines before prescribing this medication, so we do not unintentionally cause harm while looking to appease our patients who request this.
Semaglutide and Type 2 Diabetes
Introduction
In 2017, the FDA approved the semaglutide injectable (Ozempic) for treating type 2 diabetes. The drug has experienced widespread acceptance due to its positive effects on weight loss and lowering of chronic health risks. The drug has risen in popularity over the past few years, as many well-known actors/actresses/songwriters, and more came forward, publicly sharing their weight loss journey.
This rise in popularity has also resulted in significant shortages of this medication, negatively impacting the lives of the diabetic community, local pharmacies, and healthcare providers. The goal of this continuing education course is to educate and empower the healthcare provider in all aspects of this drug regimen: clinical indications, patient education, cost options, and benefit/risk analysis.
Diabetes Overview
Diabetes is a chronic medical condition. Despite advances in diet, medications, and monitoring devices, diabetes diagnoses continue to grow at staggering rates. The Institute for Health Metrics and Evaluation (IHME) reports that over 529 million people worldwide are currently living with diabetes, and that number is expected to grow to 1.3 billion in only 30 years. While the risk factors for diabetes are vast in number (poor diet, inadequate activity, obesity, sedentary lifestyles, daily stressors, and more), the sad reality is that this chronic medical condition will most likely linger on for generations to come despite our efforts to contain this health epidemic (1).
According to the latest research on diabetes, there are over 37 million people in the United States alone with diabetes as of 2022. Statistically, approximately 28 million of them have a confirmed diagnosis, while another estimated 8 million are experiencing symptoms, without an official diagnosis. Diabetes currently ranks as the 7th leading cause of death in the United States (2).

Self Quiz
Ask yourself...
- As a healthcare provider, what has been your experience with treating chronic medical conditions?
- Why do you think there is a continued increase in diabetes, despite advances in medication and monitoring devices to treat this condition?
- Are you currently offering comprehensive care to your patients, including medication, diet, and activity counseling for their chronic health conditions?
Types of Diabetes
In basic terms, diabetes is an impairment in one’s ability to either adequately produce or utilize insulin, which results in elevated levels of circulating glucose. Chronically elevated glucose levels affect blood vessels at every level, causing chronic inflammation and raising the risk of heart disease, stroke, blindness, and atraumatic amputations.
There are three main types of diabetes:
Type 1 diabetes is thought to be an autoimmune disease. Approximately 5-10 percent of people with diabetes are diagnosed with type 1 diabetes. The diagnosis usually occurs in early childhood, and results in a lifetime use of insulin to regulate blood glucose levels.
Type 2 diabetes is thought to be related to dietary and lifestyle choices. It accounts for nearly 90-95 percent of diabetes diagnoses. Usually occurring later in life (adult-elderly population), it is believed to be related to factors such as diet, activity, weight gain, and related factors. Type 2 diabetes is usually controlled by diet and exercise, in addition to oral medications, although injectable insulin may be included in the treatment plan.
Gestational diabetes refers to elevated glucose levels occurring during pregnancy for patients who are not diabetic at the onset of pregnancy. This version of diabetes usually resolves itself post-partum, although a woman may develop type 2 diabetes later in life, unrelated to pregnancy.
Type 2 diabetes in children: no longer a “later in life diagnosis”
Children are now being diagnosed with type 2 diabetes at an alarming rate. Despite widespread education and an increased awareness of diabetes, our up-and-coming generation is unhealthier than ever. Many families lack access to healthy food for their families, due to both general socioeconomic challenges and an increased rate of food insecurity. (19)
The CDC recommends care providers have resources for diabetic patients and their families, such as food and nutrition programs, budget-friendly diabetes meal plans, how to save money on diabetes care, and coping strategies for diabetes. (19)


Self Quiz
Ask yourself...
- Are you able to articulate the different types of diabetes to patients?
- What resources can you offer to the families of children with type 2 diabetes?
Diabetes Signs and Symptoms, Diagnostic Testing
There are various ways to test for diabetes. The fasting blood sugar (FBS)/ fasting glucose level is a simple way to test for diabetes.
The normal fasting glucose level is below 100mg/dl. The fasting glucose result of 100-125mg/dl indicates prediabetes and results above 126mg/dl indicate diabetes.
The hemoglobin A1C blood test is another test used to confirm the diagnosis of diabetes. The patient does not need to be fasting for this test; thus, it is easier to order this test regardless of the time of day. This blood test reflects the average glucose level over the period of 2-3 months.
The normal A1C level is below 5.7%. Test results between 5.7%- 6.4% indicate prediabetes. Test results above 6.5% indicate diabetes.
A random glucose reading above 200mg/dl, done at any time of day, indicates diabetes.
The diagnosis of diabetes is by blood tests, and for improved accuracy, should be based on two separate readings, done (at least) a day apart. In the case of fasting and random blood tests, dietary intake (large amounts of carbohydrates in a single meal) may adversely affect test results. This is not the case when using A1C testing for a confirmation diagnosis, as the results are the average of a 2–3-month span.
Target blood levels for a person with diabetes (3).
Target blood glucose levels for people with diabetes are as follows:
- Fasting glucose 80-130mg/dl.
- Postprandial blood glucose level- less than 180mg/dl
- A1C level 7-8%.
These target ranges are general guidelines. Patient-specific ranges will be dependent on a variety of factors, including preexisting comorbidities, overall health status, age, and activity levels.
The hallmark signs/symptoms of diabetes
- Polyuria- increased urination
- Polydipsia- increased thirst
- Polyphagia-increased hunger/appetite
The truth is, as healthcare providers, you will have patients who have no hallmark signs and symptoms of diabetes; the diagnosis will be found during annual preventive examinations often unrelated to any chronic disease. For this reason, many insurance companies now cover numerous preventive screenings, including diabetes screenings, as part of their wellness and prevention initiatives. These tests are often approved based on a patient's age, or preexisting conditions, rather than outright signs and symptoms.

Self Quiz
Ask yourself...
- What are the typical glucose levels for non-diabetic versus diabetic patients?
- What are the hallmark symptoms you can identify when treating a potentially diabetic patient?
Lifestyle Interventions and the Diabetes Prevention Program
The initial diagnosis of diabetes can be managed in a variety of ways, depending on the severity of the illness at the time of diagnosis. Lifestyle interventions (behavior modification education) are of utmost importance in the care and management of people with diabetes. Research over the past few decades has consistently shown that such interventions have immense positive effects on the successful long-term management of diabetes.
The official Diabetes Prevention Program was created in 2010 (4) and confirmed the effects of lifestyle interventions in the management of diabetes: Lifestyle interventions decreased the incidence of type 2 diabetes by 58% compared with 31% in the metformin-treated group. Thus, these findings now serve as the blueprint, if you will, for all-inclusive, patient-specific disease management guidelines. These lifestyle interventions will be discussed in detail later in the program.
Additional Resources on Diabetes Prevention

Self Quiz
Ask yourself...
- How do lifestyle interventions compare to other kinds of treatment for patients with type 2 diabetes?
Semaglutide
Semaglutide is an injectable drug used in the treatment of type 2 diabetes. It was approved by the FDA in May of 2017.
It is a once-a-week injectable and belongs to the drug class known as glucagon-like peptide-1 receptor agonists (GLP-1RAs) (5). It has been referred to as a “miracle weight loss drug” among those who are living with obesity, despite frequent side effects, unusually high out-of-pocket costs, drug shortages, and weight regain when attempting to stop using the medication.
GLP-1 receptor agonist: Hormone Review
GLP-1 RAs are a class of medications used to treat Type 2 diabetes, and in some cases, obesity treatment. They are also known as GLP-1 receptor agonists, incretin mimetics, and GLP-1 analogs.
Ghrelin and Leptin (6)
Ghrelin and Leptin are two hormones that greatly influence appetite and the sensation of fullness. Often referred to as the “hunger hormone.” Ghrelin is responsible for many functions, including playing a key role in metabolism through glucose and insulin regulation.
Ghrelin, produced in your stomach, signals your brain when you are hungry, and results in increased food intake.
Leptin, conversely, is produced in your fat cells, and signals to the brain when you have eaten enough (by a decrease in appetite).
Glucagon-like peptide-1 receptors
Known as GLP1 receptors, Glucagon-like peptide-1 receptor proteins are located in the beta cells of the pancreas as well as in the neurons in the brain. GLP-1 receptors are involved in the regulation of blood glucose levels and affect the secretion of insulin. These cells encourage the release of insulin from the pancreas, increase the volume of beta cells, and reduce the release of glucagon. In doing so, they increase the feeling of fullness during and between meals, suppressing the appetite and slowing gastric emptying.

Self Quiz
Ask yourself...
- What are some problems patients might face if they choose to take semaglutide?
- How do Ghrelin and Leptin relate to a patient's appetite?
What is meant by receptor agonist and antagonist?
The term agonist refers to any substance that mimics the actions of a hormone in producing a specific response: a receptor antagonist blocks a response from occurring.
Opioids are examples of receptor agonists in that they produce responses such as analgesia.
Naloxone/Narcan is an example of a receptor antagonist, in that it binds to a receptor site and decreases/blocks a response from occurring.
Semaglutide mechanism of action (7)
GLP-1 agonists work in several ways to positively affect glucose levels. Their mechanism of action includes the following:
- Increasing (stimulating) insulin secretion by the pancreatic beta cells.
- Decreasing the production of glucagon, a hormone that raises blood glucose levels
- Decreasing (slowing) gastric emptying
- Decreasing appetite (and thereby reducing food intake) by creating a sensation of stomach fullness
Through these mechanisms of action, semaglutide results in a lowering of serum glucose/A1C levels, which lowers the risk of cardiovascular events. Studies have also shown that semaglutide resulted in weight loss (approximately 8-14 pounds on average {dose dependent results}.

Self Quiz
Ask yourself...
- What is the difference between an agonist and antagonist substance?
- How much weight do patients lose, on average, when taking semaglutide?
Side Effects of Semaglutide
Common side effects of semaglutide (8)
Common side effects may include any of the following:
- Nausea and vomiting
- Headache
- Diarrhea and stomach pain
- Upset stomach, indigestion, constipation, flatulence
These side effects usually subside within a few weeks, as the patient becomes acclimated to the medication.
Serious side effects of semaglutide
- Hypoglycemia- enhanced/worsened when used in combination with other diabetes medication. Symptoms may include drowsiness, confusion, weakness, irritability, and headache.
- Symptoms may include abdominal pain and distension, nausea and vomiting, fever, and back pain.
- Diabetic retinopathy. Symptoms may include blurred vision, vision loss, and diminished night vision.
- Kidney damage/injury/failure. Symptoms may include fatigue, nausea, diminished urine output, confusion, and edema of extremities.
- Gallbladder disease. Symptoms may include gallstones, abdominal pain, nausea and vomiting, and poor appetite.
Black Box Warning (9)
Semaglutide has a Black Box Warning for thyroid cancer. This is the most serious warning from the Food and Drug Administration (FDA) and is intended to alert consumers to the potential risks of a medication. This black box warning was issued when research found that the drug increased the risk of thyroid tumors in animals.
It is not known if semaglutide actually causes tumors in humans.
Contraindications
- Semaglutide is contraindicated in people with a personal or family history of MTC (medullary thyroid cancer) or in patients with multiple endocrine neoplasia syndrome type 2.
- Known hypersensitivity to semaglutide or any of the product components
Cautions
As noted under “serious side effects”, there have been reports of new illnesses or worsening of existing health conditions occurring “post-marketing”. Thus, healthcare providers are strongly encouraged to continue ongoing surveillance of any patients on semaglutide therapy. In addition, there is insufficient data available regarding the use of semaglutide by pregnant women. Women are therefore highly encouraged to stop any treatment with semaglutide for at least 2 months prior to a planned pregnancy.

Self Quiz
Ask yourself...
- Can you name the 4 common side effects of semaglutide?
- What is the most severe warning associated with semaglutide?
Dosing
Semaglutide is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus (T2DM). It is looked upon favorably to reduce the risk of cardiovascular events in adults with T2DM and a preexisting history of cardiovascular disease. This drug is FDA-approved for use in people with diabetes, with a BMI of 27% or higher (a BMI of 25-29.9% is considered overweight).
Semaglutide (Ozempic) is available as an injectable prescription medication. Doses include 0.5mg, 1mg, or 2 mg, once weekly.
The injection should be administered subcutaneously to the abdomen, thigh, or upper arm. Injection sites should be rotated, and given as a single injection.
Start at 0.25 mg once weekly. After 4 weeks, increase the dose to 0.5 mg once weekly.
- If additional glycemic control is needed, increase the dose to 1 mg once weekly after at least 4 weeks on the 0.5 mg dose.
- If additional glycemic control is needed, increase the dose to 2 mg once weekly after at least 4 weeks on the 1 mg dose
Administer once weekly at any time of day, with or without meals. The maximum dose recommendation is 2mg/weekly once weekly.
Note: The initial 0.25-mg dose is intended for treatment initiation and is not effective for glycemic control
Missing Dose Guidelines
- If the missed dose is ≤5 days: Administer dose as soon as possible
- If missed dose >5 days: Skip the missed dose and administer the next dose on the regularly scheduled day; patients can then resume their regular once-weekly dosing schedule
Administration Day Guidelines (10).
The administration day each week can be changed, if necessary, as long as the time between 2 doses is at least 2 days (>48 hours)
Dose Availability (packaging)
- 2mg/1.5mL (1.34mg/mL); delivers doses of 0.25mg or 0.5mg per injection or four to eight doses per injection pen
- 4mg/3mL (1.34mg/mL); delivers 1mg per injection or 4 doses per injection pen
- 8mg/3mL (2.68 mg/mL); delivers 2mg per injection or 4 doses per injection pen
Treatment Goals- Effects on A1C and Weight (11)
A majority of adults who were placed on injectable semaglutide for diabetes management achieved a target A1C under 7% and were able to maintain it.
- Dose specific effects on A1C were as follows:
- 0.5mg dose injection yielded a 1.4% decrease
- 1.0mg dose injection yielded a 1.6% decrease
- 2.0 mg dose injection, in combination with diabetes pills, yielded a 2.1% decrease in A1C.
Adults taking semifluid injectables for diabetes management also noted weight loss.
- 8-pound weight loss reported with 0.5mg dose injection
- 10 pounds weight loss reported with 1.0mg dose injection
- Up to 14 pounds of weight loss reported with a 2.0mg dose injection


Self Quiz
Ask yourself...
- What should you tell a patient if they miss their injection by more than 5 days? What if it has been less than five days?
Prescribing insights: Long-Term therapy for a chronic condition?
Semaglutide is viewed favorably as a treatment option for Type 2 diabetes. It appears to lower A1C levels and body weight in the majority of patients, lowering their risk of future cardiovascular events.
The question of long-term medication use, for a chronic health condition, is being heavily discussed in the media. While a percentage of people can decrease or eliminate the need for chronic medications through significant lifestyle changes, there have been reports of weight gain in those who stopped taking this injectable medication.
Without intense lifestyle behavior modification education, there is a heightened risk of weight regain in the absence of such medications. Leaders in the treatment of obesity and related illnesses have commented that this drug is intended for long-term use.
Examples of this include the following:
“GLP-1 medications [like Ozempic] are designed to be taken long-term... They are chronic medications for the treatment of chronic conditions (both diabetes and obesity) (12)". - Christopher McGowan, M.D., a gastroenterologist specializing in obesity medicine and endobariatrics
“As with many chronic conditions, most people who use the drugs for diabetes or weight loss will need to keep taking them to keep benefiting from them. Depending on your individual situation, and without sustained lifestyle changes, it is likely you would need to be on these medications indefinitely to maintain weight loss (13)." - Dr. Cecilia Low Wang, a UCHealth expert in endocrinology, diabetes and metabolism.

Self Quiz
Ask yourself...
- Is semaglutide considered to be a long-term treatment for type 2 diabetes?
Cost Concerns
At this time, injectable semaglutide, FDA-approved for the treatment of Type 2 diabetes, has a self-pay price tag of $935.77 per month (4 injections). With FDA approval, many people with diabetes, insured under commercial plans, receive the drug for the cost of their copay. Those patients without coverage may use pharmacy discount cards that reduce the price, on average, to $814.55/month.
The following links are available to familiarize yourself with patient assistance programs related to semaglutide injectables.
Semaglutide Cost Savings Programs
The following links are provided to explore various semaglutide cost savings programs.

Self Quiz
Ask yourself...
- What resources can you offer patients who are struggling to pay for semaglutide?
Emerging Concerns: Semaglutide and gastroparesis
In August 2023, a first-of-its-kind lawsuit was filed in Louisiana, against the makers of semaglutide. The lawsuit states the makers of this injectable drug did not adequately warn patients about the risk of severe gastrointestinal issues/possible gastroparesis.
The plaintiff in this case had used both Ozempic and Mounjaro and experienced repeated episodes of severe gastrointestinal events, warranting trips to the emergency room and additional medications to alleviate her symptoms (14). While this lawsuit is in the developing stages, it bears mentioning in terms of concerns over long-term usage of the drug and possible complications.
While the drug labeling for semaglutide (Ozempic) does not specifically mention gastroparesis, the semaglutide/Mounjaro drug label does state that the drug has not been studied in patients with severe gastrointestinal disease and is therefore not recommended in these patients.
Up to 50% of people with diabetes have some degree of delayed gastric emptying, but most have no digestive symptoms or have only mild symptoms. For some people with diabetes, problems managing blood glucose levels may be a sign of delayed gastric emptying (15).
Healthcare providers should evaluate all patients with diabetes for possible symptoms of underlying gastroparesis, such as the feeling of fullness shortly after beginning a meal, or the inability to finish a regular meal. Other symptoms of gastroparesis may include abdominal pain, nausea, bloating, vomiting, and anorexia.
Diabetes and gastroparesis
Uncontrolled or poorly controlled diabetes can affect nerve endings systemwide. Diabetes is a very common cause of gastroparesis. Although the condition is rare it occurs more often in people with chronic conditions such as diabetes, autoimmune diseases, and nervous system disorders. Nerve endings are injured or damaged, cease to function properly, and result in delayed gastric emptying. The delay in gastric emptying can cause various symptoms, such as nausea, vomiting, bloating and distension, abdominal pain, and poor appetite.
In addition to underlying medical conditions, some medications may cause symptoms of gastroparesis (delays in gastric emptying and overall gastric motility. These medications include narcotics, antidepressants, and anticholinergics.
Left untreated, diabetic gastroparesis may lead to malnutrition, electrolyte imbalances, and poor glucose management and control.

Self Quiz
Ask yourself...
- Why should nurses prescribing semaglutide watch out for symptoms of gastroparesis?
- What do you think are some ethical issues with semaglutide use for weight loss?
Diabetes Lifestyle changes: Patient education (16)
- Weight Management
- Healthy Eating
- Physical Activity
- Smoking Cessation
- Stress Management
The importance of patient education regarding lifestyle changes is a priority. As with any chronic medication condition, the patient and their family/support system must be given every opportunity to educate and empower themselves on self-management of their disease process. Patients must be given the benefit of the doubt that they can indeed embrace their health and well-being and work with their healthcare provider in maximizing their health outcomes.
For diabetes mellitus, numerous lifestyle behaviors should be addressed and actively worked on, so that the patient receives the maximum health benefits. The following lifestyle behaviors are in no particular order; they all warrant discussion at every office visit.
Diet
A person with diabetes should be educated on the effects of food and nutrition on their glucose level. Referrals to a dietitian/nutritionist or Certified Diabetes Care Education Specialist (CDCES) should be considered a top priority. Well-balanced nutritional intake, appropriate carbohydrate awareness, calorie monitoring if weight loss is appropriate to your specific patient) and medication/food interactions are all essential aspects of dietary lifestyle education. Many commercial insurance plans, as well as hospital community outreach programs, offer diabetes self-management classes.

Activity (17)
The CDC recommends a target goal of 150 minutes weekly, Patients should be educated on the positive effects of daily activity on overall health and well-being, stress management, and metabolism. Patients should find activities they are genuinely interested in, involve family and friends, and slowly build greater endurance through increased intervals of longer duration.
Sleep hygiene (18)
Patients should be educated on the positive effects of a good night’s sleep. The aim should be approximately 7-8 hours of restful sleep. Electronics should be powered down and (optimally) removed from the bedroom. A dark, well-vented, cool room temperature is encouraged, and large meals and late-evening caffeine should be avoided.
Medication adherence/ literacy
Medication education is critical to the health and well-being of a patient. Routine education of the patient, and family members or support systems when available, should be supportive and patient-specific. Patients should be assessed on language barriers, literacy issues, and related comprehension concerns. Medication education should include effects, side effects, treatment goals, and sick day management. Emergency care issues should also be discussed. Any monitoring equipment (continuous glucose monitors, accuchecks, lancets) should be reviewed with patients and confirmed with return verbalization and demonstration.
As discussed in this course, patients with chronic diseases must learn self-management techniques to optimize their health and well-being. They must become confident in their understanding of their disease process and take ownership of their health. In doing so, they minimize the risk of long-term complications, improve their self-worth, and actively invest (both time and money) in their future.

Self Quiz
Ask yourself...
- How does sleep, diet, and activity level affect the treatment of type 2 diabetes?
Ozempic Case Study
- 52-year-old female
- Height 67 inches
- Weight 225 pounds
- B/P 138/84, Heart rate 76 NSR
- BMI 35.2%
- Nonsmoker, occasional social drinker
- Multiple attempts at dieting without success.
- Diagnosed T2DM approx. 6 months ago current A1C 7.5%; initial medication Metformin 500mg BID tablets; tolerated well. No GI upsets.
Today’s appointment is for evaluation and additional medication consideration (the patient requested this appointment)
The patient was diagnosed with T2DM approximately 6 months ago. Initial A1C 8.0%. Current A1C 7.7%
Despite an improved diet and adherence to the medication regimen, the patient voiced frustration at the lack of weight loss. Requesting additional medication. Has a neighbor friend who began injectable Ozempic and is having “really great results with it. I want to start on it as well”.
- What are your thoughts on prescribing semaglutide injectable for this patient?
- What objective health data points should be taken into consideration regarding prescribing semaglutide for this patient?
The patient has expressed frustration that despite taking her medications and adjusting her diet, she has not lost any weight in the past 6 months. She has “heard from her neighbor friend that the weight just melts off immediately” and she is ready to start this medication.
- What concerns do you know about this patient's understanding of weight loss as it relates to semaglutide?
- What prescribing information, specific to semaglutide and weight loss, could you share with your patient regarding realistic weight loss targets?
- In addition to teaching your patient proper injection technique for the use of semaglutide, what other lifestyle education behaviors should you discuss at this point?
- What information should you share with your patient regarding the long-term use of semaglutide and the potential risks of stopping this medication (as it relates to weight regain)?
Your patient decides to go ahead with the semaglutide regimen.
- What are some patient education guidelines regarding common side effects of this medication?
- How often is the dose increased? What is the maximum dose this patient can receive weekly?
Your patient wants to know how long she will be taking this medication.
- What talking points will you cover regarding the long-term use of this medication?
- How do you best prepare this patient for long-term success with this medication?
- What lifestyle behavior modification education would you discuss with your patient, to give her the best chance at successfully managing her diabetes?
Medication Assisted Treatment
Introduction
Medication Assisted Treatment (MAT) is a treatment modality for substance use disorders. It combines counseling and behavioral therapies for addiction with medications used carefully to reduce the physical symptoms of cravings and withdrawal and assist clients in the recovery process. With half of people 12 and older reporting use of an illicit substance at least once and 21 million Americans experiencing addiction, this is an important and relevant topic (4).
Historically, an intense stigma is attached to both addiction and some of the medications used to treat addiction. A thorough understanding of substance use disorders, available MAT therapies, and care of affecting clients are essential topics for nurses to be familiar with, particularly those working in psychiatry, pain management, or addiction medicine.
Overview of Addiction and Substance Abuse:
Drug and alcohol abuse and addiction are chronic, complicated issues involving persistent changes to the brain. There is a stigma or misunderstanding that people with substance abuse disorders can stop any time they want to or lack the willpower or moral fortitude to stop using. This is entirely untrue, and even people who are "recovering" and have not had any drugs or alcohol in years can easily relapse into addiction once those brain changes have occurred (5).
When a person uses drugs or alcohol, the brain's reward center is flooded with dopamine. This provides a "buzz" or pleasurable sensation that may create the desire to use more of the same substance. Over time, and with regular use of the substance, the brain becomes accustomed to the flooding of dopamine and reduces the reward response, a process known as tolerance.
It will now take the same person a more significant amount of the substance to achieve the same "buzz" or "high" they used to feel. This process can also dull the pleasure response to activities not involving substance use, such as food, socialization, or sexual activity. Over time, the chemical changes in the brain can progress to include decreased functioning of learning, decision-making, judgment, response to stress, memory, and behavior (5).
To understand substance abuse disorders, it is first essential to understand some basic definitions. These terms are sometimes used interchangeably, but they mean different things and represent different stages of disease.
Definitions
Substance Use: Substance use is any consumption of drugs or alcohol, regardless of frequency or amount. An occasional glass of wine or taking an edible at a party is an example of substance use. Substance use does not cause problems or dependency in many people (5).
Substance Abuse: Substance abuse is the continued use of drugs or alcohol, even when they do cause problems. Conflict or problems at home, school, work, or legal issues related to the use of drugs or alcohol are signs of abuse. For example, being sent home from school for smoking in the bathroom or failing a drug test at work (5).
Substance Dependence or Addiction: Dependence and addiction can be used interchangeably or is sometimes called substance use disorder. Addiction occurs when a person cannot stop drinking or using drugs despite creating problems in their life. People who are addicted may experience cravings until they use a specific substance, or they may experience uncomfortable physical symptoms, known as withdrawal if they do stop (5).
The American Psychiatric Association (APA) utilizes the following criteria to diagnose clients who suffer from addiction. The more criteria a client answers yes to, the greater their problem with substance use.
Six or more positive criteria are indicative of addiction.
- Using substance in more significant amounts or for more extended periods than intended
- Trying to stop using but being unable to
- Increased amounts of time getting, using, or recovering from use of the substance
- Experiencing cravings or urges to use.
- Continuing to use the substance despite problems with relationships or social situations.
- Missing work, social, or recreational obligations or activities because of substance use
- Participating in risky behavior because of substance use
- Continuing to use the substance despite psychological or physical health problems.
- Needing to use more substance over time to achieve the desired effect.
- Experiencing withdrawal symptoms when stopping the substance (1).


Self Quiz
Ask yourself...
- Do you know anyone who suffers from a substance use disorder?
- Think about your biases (thoughts, opinions, attitudes) about addiction. Does any of the information above conflict with those biases?
Substance Abuse Statistics
Many factors go into gathering data on substance abuse disorders, from underreporting, the nuance between use, abuse, and addiction, and the large variety of substances available, with the legality of some substances varying by state or age.
The statistics below from 2020 are not meant to be an exhaustive list of substance use disorders in this country but rather an overview of some of the more prevalent addiction-related issues.
- 50% of people 12 years and older have used an illicit substance at least once.
- 5% of Americans 12 years and older have used drugs within the last month.
- This is a 3.8% increase from the previous year.
- About 50% of Americans 12 and over drink alcohol
- 4% of those people have an alcohol use disorder.
- About 20% of Americans use tobacco products or vape
- 18% of Americans over 18 used marijuana in the last 12 months
- 30% of those have some level of misuse or addiction.
- Marijuana is commonly involved in polysubstance use, paired with alcohol or other drugs.
- 7% of Americans over 12 misused opioids in the last 12 months
- 96% of those used prescription pain relievers
- Opioid prescriptions peaked in 2012, with 81.3 prescriptions per 100 people.
- The rate has declined recently due to increased attention to this crisis.
- In 2018, the rate was down to 51 prescriptions for every 100 people
- Fentanyl is now rising as a new and deadly concern.
- 5 million prescriptions were written for fentanyl in 2015.
- Fentanyl is involved in 53% of overdose deaths.
- 7% of all Americans misuse a prescription drug.
- 1% of those misuse stimulants
- 2% of those misuse sedatives
- 5% misuse painkillers
- Over 70,000 drug overdose deaths occur annually in the United States (4)
Risk Factors
A combination of factors is involved in the risk of addiction, and no one factor can determine if someone will develop addiction or after how many uses this will occur.
The addiction process does occur more easily or progresses more rapidly for people with certain risk factors, including:
Genetics
There is a strong genetic correlation with addiction, indicating that biology plays a significant role in the disorder. Family history of addiction, gender, ethnicity, and comorbid mental health conditions can all influence the risk of addiction. (5)
- Children of addicts are eight times more likely to develop an addiction at some point.
- In 2020, among those using illicit or misusing prescription drugs, 22% were male and 17% female.
- Only 20% of users in drug treatment programs are women.
- 9% of people with substance abuse disorders also have at least one mental health disorder (4)
Environment/Non-Genetic Demographics
The attitudes about drugs and alcohol from those in a person's network and life experiences play a role in the risk of addiction. Substance use among friends, family, or coworkers increases the risk that a person will also use substances. Exposure to substance use from a young age relaxed parental attitudes about substance use, and peer pressure from friends can increase the risk. Certain stressful life circumstances such as veteran status, history of sexual or physical assault, or being part of the LGBTQ community can also increase risk. (5)
- 20% of people in urban areas used illegal drugs in 2020 compared to 5% in rural locations.
- 51% of Americans with an illegal pain relief medication obtained it from a friend or relative.
- 7% of LGBTQ Americans abuse illicit drugs.
- 2% of LGBTQ Americans abuse alcohol.
- 7% of Veterans abuse illicit drugs.
- 80% of Veterans abuse alcohol (4)
Developmental Stage
Substance use at any age can lead to addiction, but children and teens are at particular risk due to their underdeveloped brains. The parts of the brain responsible for decision-making, risk assessment, and self-control do not fully develop until the early 20's, putting teenagers at increased risk of dangerous behaviors. In addition, the effects of drugs and alcohol on the developing brain may mean that those parts of the brain never fully develop at all for teens with substance abuse disorders. (5)
- 70% of users who try an illegal substance before age 13 will develop a substance use disorder within the next seven years.
- This is for only 27% of people who first try an illegal substance after age 17.
- 47% of youths report trying an illegal substance by the time they graduate high school (4)

Self Quiz
Ask yourself...
- Why do you think medication alone is not an adequate treatment for substance abuse disorders?
- Is MAT something you have heard of before? Why do you think it is relatively uncommon despite being around for decades?
Overview of Medication Assisted Treatment (MAT)
Treatment of substance abuse disorders is a complex and often tumultuous process. The nature of the brain changes that occur during addiction means that a person is never entirely "cured" but will always be considered "recovering" as the risk for relapse is always present. Effective treatment must be multifaceted and often involves removing triggers (such as people, places, and stressors) that may prompt a person to use again behavioral therapy, and medications to curb withdrawal symptoms and reduce cravings.
Medication Assisted Treatment (MAT) is a treatment that involves FDA-approved medications, in combination with behavioral therapy, in the recovery process for substance abuse disorders. Several medications are available for MAT, and evidence continues to emerge that the treatment is highly effective if used correctly.
However, it is a vastly underused and understudied treatment modality. MAT has been available in some form for over 50 years but is just starting to gain traction among the medical community (and policymakers) in recent years, with the federal government calling for more research and increased accessibility for the treatment (8).
The height of the opioid crisis in the last several years has highlighted the magnitude of drug addiction and deaths in the United States, bringing renewed attention to MAT as a treatment option. So, how does MAT work? Prescription medication is given to both stimulate the receptors seeking the abused substance and block the drug's euphoric effects.
Over time, this normalizes brain chemistry and helps the person break the habit of using without the discomfort of cravings and withdrawal symptoms. Gradually, the prescription medication dosage is reduced, all the while in conjunction with behavioral therapy and lifestyle changes, and eventually, the client should be able to stop the medication altogether, often within 1-3 months (8).
MAT does require close supervision by a trained medical professional and an appropriate facility for treatment. It can be done on an inpatient, partial inpatient, or outpatient basis. There may be side effects to the medication, and there is a risk of misusing or developing addiction to the new drug, though the successful outcomes often outweigh this risk. Clients must also participate in behavioral therapy for a comprehensive and effective treatment plan. As with any treatment regimen, careful consideration of the client's history and circumstances is essential (8).


Self Quiz
Ask yourself...
- Why do you think medication alone is not an adequate treatment for substance abuse disorders?
- Is Medication Assisted Treatment (MAT) something you have heard of before? Why do you think it is relatively uncommon despite being around for decades?
Pharmacokinetics
Currently, there are three medications with FDA approval for MAT: buprenorphine, methadone, and naltrexone. Each will be discussed in depth below.
Buprenorphine
Mechanism of Action and Metabolism
Buprenorphine is an opioid partial agonist, acting on the same receptors as other opioids but with weaker effects. It can be used for the treatment of misuse of opioids, including:
- Heroin
- Fentanyl
- Oxycodone
- Hydrocodone
- Morphine
- Methadone (3)
Opiate receptors are G-protein coupled receptors (GPCRs) with four major types: Mu, Delta, Kappa, and opioid receptor like-1 (ORL1). Stimulation of these receptors results in varying levels of the following effects:
- Euphoria
- Relaxation
- Pain relief
- Sleepiness
- Sweating
- Constipation
- Impaired concentration
- Reduced sex drive (3)
Buprenorphine has a high affinity to the Mu-opioid receptor and is a partial agonist at this site, causing reduced opioid effects with a plateau or ceiling at higher doses. This limits dangerous effects and makes overdose unlikely. It also has slow dissociation from the site, allowing milder and more easily tolerated withdrawal effects compared to full agonists like morphine and fentanyl. Buprenorphine is also a weak kappa receptor antagonist and delta receptor agonist, reducing the craving sensation and improving tolerance to stress (3).
Buprenorphine has poor bioavailability when given orally due to the first-pass effect, where most of the drug is broken down in the liver and intestines. Because of this, sublingual or buccal are the preferred routes of administration and the most common forms in which the drug is manufactured. Transdermal patches and IV and IM forms exist, though not for use in MAT (3).
CYP34A enzymes break down buprenorphine, so other drugs, such as ketoconazole, may inhibit metabolism and increase available levels of buprenorphine. CYP34A inducers such as carbamazepine, topiramate, phenytoin, and barbiturates may speed metabolism and lower available levels. Once broken down, the med takes the form of norbuprenorphine and is excreted in the feces (3).
Available Forms
Buprenorphine is available by itself and with naloxone (in a 4 to 1 ratio). However, in oral form, naloxone is not readily absorbed, and buprenorphine is the only genuinely active ingredient. This combination is beneficial should clients try to inject their buprenorphine to get high; naloxone is a fast-acting opioid antagonist that is active when used intravenously and would block the opioid effect of buprenorphine, rendering it useless for recreational use and ensuring it has no street value.
The currently available preparations of buprenorphine for MAT include:
- Generic Buprenorphine/naloxone sublingual tablets
- Subutex - Buprenorphine sublingual tablets
- Suboxone - Buprenorphine/naloxone sublingual films
- Zubsolv - Buprenorphine/naloxone sublingual tablets
- Bunavail - Buprenorphine/naloxone buccal film (3)
Sublingual products dissolve within 2-10 minutes. Bloodstream absorption begins quickly, bypassing the first pass effect. Buprenorphine has a slow onset of action, peaking about 3-4 hours later. Metabolism is also slow, with the half-life lasting anywhere from 25 to 70 hours (an average of about 38 hours). This long half-life means the drug can be spaced out to every other day administration once weaning begins (3).
Dosing and Monitoring
Clients prescribed buprenorphine must stop using opioids for at least 12 to 24 hours before the first dose; this varies depending on which opioid they are stopping. For short-acting opioids like heroin and oxycodone, buprenorphine may be started 6-12 hours after the last dose. With longer-acting opioids such as morphine or extended-release preparations of oxycodone, buprenorphine should be delayed for about 24 hours. For the longest action opioids, fentanyl patch, 48 -72 hours must be between the last dose and buprenorphine initiation (3).
This initiation schedule means clients will be in the early stages of discomfort and withdrawal. Administration of buprenorphine when clients still have opioids in their bloodstream will lead to competition for receptor sites, rapidly replacing the opioid with buprenorphine and causing acute and more severe withdrawal symptoms.
Depending on the severity of a client's addiction, they may complete the first step of abstaining and withdrawal in an inpatient setting. Once the initial withdrawal symptoms have passed and the initial dose of buprenorphine has been given, the client may be discharged home to continue buprenorphine initiation on an outpatient basis (3).
Initial doses are typically 2-4mg, with up to 4mg given to clients used to higher potency or larger doses of opioids. The dose is gradually increased to meet the client's individual needs, with a maximum dosage of 24mg per day. The average client requires 8-12 mg per day and can reach this dose within the first 2-4 days. It is recommended that doses be supervised by a pharmacist at the dispensing pharmacy for the first two months of treatment to ensure compliance and clients are less likely to relapse (3).
The length of treatment with buprenorphine depends on each client's case and, for some, may be indefinite. Clients who do wish to wean off buprenorphine can begin the process once they are stable and experiencing few or no cravings, and a minimum of 8 weeks from treatment initiation. Doses are moved to alternating days and eventually discontinued altogether (3).
Side Effects and Contraindications:
As with any medication, there are potential side effects, including:
Common Side Effects
- Nausea
- Vomiting
- Drowsiness
- Dizziness
- Headache
- Memory loss
- Sweating
- Dry mouth
- Miosis
- Postural hypotension
- Sexual dysfunction
- Urinary retention
Serious side effects
- CNS depression
- QT prolongation
- Reduced seizure threshold
- Potential for abuse or overdose (3)
Buprenorphine is contraindicated for clients with a past hypersensitive reaction to it. It should be used cautiously for clients with respiratory suppression, older adults, or for those with liver pathologies. Regular monitoring of liver enzymes via lab work is essential (3).
It is a Category C medication for pregnancy, and the risks versus benefits should be carefully weighed. Buprenorphine does cross the placenta and increases the risk of withdrawal symptoms and neonatal abstinence syndrome (NAS) after delivery. However, for pregnant clients with the highest risk of relapse and abuse of opioids, evidence does support that continuation of buprenorphine during pregnancy may improve maternal and fetal outcomes (3).
Buprenorphine may be abused by crushing tablets, snorting the powder, or dissolving it into an injectable solution. Safety measures against this include supervised administration by a pharmacist and the addition of naloxone, which blocks the buprenorphine effects. While the effect ceiling of buprenorphine makes overdose difficult, combining the drug with benzodiazepines, alcohol, or other drugs can compound the CNS depressant effects and increase the risk of overdose (3).
Clinicians need to have a comprehensive health history of clients before initiating buprenorphine so that all risks and potential interactions can be addressed appropriately.
Role of the Pharmacist
Pharmacists play a significant role in the success of MAT involving buprenorphine. Outpatient doses are monitored by the dispensing pharmacist daily, with at-home quantities being allowed on a limited basis (such as weekends or travel) and only for the most motivated and compliant clients. Vital signs are collected before each dosage, with careful monitoring for hypotension or bradypnea. The dose may be skipped for clients who experience excessive side effects, and the client can return the next day for their dose.
Clients presenting with signs of overdose (usually to the ED) may receive naloxone, which will reverse overdose symptoms within 1 hour. Overdose symptoms include dizziness, pinpoint pupils, hypotension, bradypnea, hallucinations, seizure, or unconscious state.
If a client misses a dose, does not show up for it, or is experiencing significant side effects from buprenorphine, the prescribing clinician should be notified so that the treatment plan can be revisited and revised if needed (3).
Considerations for the Prescriber
When considering which medication to prescribe for MAT, prescribers should understand that buprenorphine offers advantages over methadone.
- Lower risk of abuse
- Safer, including at higher doses.
- Therapeutic dose achieved quickly.
- Easier to taper.
- Can be obtained from any provider rather than a methadone clinic.
- Less stigma
The cost of a 30-day supply is around $300. Buprenorphine/naloxone combinations are a little more expensive at $400/month. While prior authorization is usually required, most commercial insurance and state Medicaid programs will cover the medication.
Buprenorphine is a Schedule III Controlled Substance; however, recent federal regulations have been aimed at approving access to MAT, and any provider with an active DEA license may prescribe buprenorphine as allowed by state regulations. Specialized clinics are not required (as they are with methadone), and it is dispensed at regular pharmacies.
Prescribers are encouraged to participate in additional training about MAT with buprenorphine, but it is not required. Detailed documentation must be completed, including the reason for prescribing, start and end dates of treatment, the pharmacy used, the credentials of who will supervise administration, and frequency of follow-up and compliance monitoring. The sublingual and buccal routes are the only forms of medication used for MAT; patches, IM, and IV preparations are not routinely used for MAT.
The success of buprenorphine treatment depends on the client's education. Addiction potential, risk of combination with other CNS depressants, and side effects vs. signs of overdose should all be discussed with clients and their support system (3).

Self Quiz
Ask yourself...
- Given the nature of substance abuse disorders, why do you think including an opioid antagonist like naloxone in preparations of buprenorphine is necessary for safety and compliance?
- What challenges do you see with a medication needing to be administered daily with pharmacist supervision?
- What are the risks of buprenorphine being given without this supervision?
- Consider the possible pros and cons of taking a medication like buprenorphine during pregnancy. Also, consider the risks of NOT taking the drug during pregnancy when a substance use disorder is present.
Methadone
Mechanism of Action and Metabolism
Methadone is a synthetic opioid and a full agonist of the Mu-receptor site, stimulating the same effects as opioids.
- Euphoria
- Analgesia
- Sedation
It can be used as a potent analgesic for pain not responding to traditional medications, such as in clients with cancer or terminal illness, as well as for MAT and neonatal abstinence syndrome (NAS).
For this course, it will be discussed as a MAT agent, used in treatment for clients addicted to opioids such as:
- Heroin
- Fentanyl
- Oxycodone
- Hydrocodone
- Morphine
- Hydromorphone (2)
Methadone is a full agonist at the Mu-receptor, meaning it is a more potent and more easily addictive medication than partial agonists like buprenorphine. Methadone has a long half-life (8-60 hours), occupying the Mu-receptors and blocking short-acting opioids from making a client high. The longer half-life also leads to less severe cravings and withdrawal symptoms. Methadone is also an antagonist to the N-methyl-d-aspartate (NMDA) receptor, which adds to its pain relief action (2).
It has high oral bioavailability, is active in the bloodstream within 30 minutes of ingestion and remains elevated for around 24 hours. It is broken down via CYP3A4 and CYP2B6 enzymes and metabolized through the liver, making it a good option for clients with renal problems.
Medications such as ciprofloxacin, benzodiazepines, fluconazole, cimetidine, and fluoxetine may slow methadone metabolism, increasing the available drug and the side effects of overdose risk. Other medications may speed metabolism and decrease the effects of methadone, including phenobarbital, phenytoin, rifampin, ritonavir, and carbamazepine (2).
Available Forms
Methadone is available in many forms, including oral, IM, subcutaneous, IV, and intrathecal, though only the oral is typically used for MAT.
- Methadone - tablets
- DISKETS - dispersible/dissolvable tablet
- Methadone HCL Intensol - 10mg/ml suspension
- Methadone - dispersible tablet (2)
Dosing and Monitoring
Oral dosing is initiated at 30-40 mg/day with a slow titration of 10-20 mg/week until the optimal dosage is reached. The optimal dosage varies by client and depends on the drug they are replacing, tolerance to opioids, and side effects experienced. A dosage between 80- 150 mg/day is the typical goal. (2)
If parenteral methadone is given, it is usually 50%-80% of the oral dosage.
Blood sugar, EKG, and methadone blood levels should be checked regularly, every week for higher-risk patients, and every 3-6 months for those in good health and compliance. The target methadone blood level is around 400 ug/ml (2).
Side Effects and Contraindications
Potential side effects are directly related to stimulation of the opioid receptors and include:
- Diaphoresis
- Flushing
- Pruritus
- Nausea
- Dry mouth
- Constipation
- Sedation
- Lethargy
- Respiratory Depression
- QT prolongation
- Hypoglycemia (2)
Methadone should be considered with a comprehensive view of a client's health history and other medications. Clients with CNS-related disease processes (trauma, increased ICP, dementia, or delirium) must be monitored closely or have other medication considered.
Methadone should not be used simultaneously as other opioids, benzodiazepines, alcohol, or antipsychotics due to increased CNS effects. Methadone is a Pregnancy Category C medication, and risks versus benefits should be weighed carefully. Infants exposed to methadone in utero are at increased risk of NAS after delivery (2).
Overdose can occur, and clients and support systems should be educated on signs of overdose.
- Lethargy
- Somnolence
- Stupor
- Coma
- Miosis
- Bradycardia
- Hypotension
- Respiratory sedation
- Cardiac arrest
Naloxone is used to reverse overdose (2).
Considerations for Prescribers and Clinics
Methadone is a Schedule II Controlled Substance, meaning it has a high abuse potential and must be carefully monitored. The Prescription Drug Monitoring Program (PDMP) is an electronic database used nationwide to register the distribution of controlled substances so that clients do not seek care at multiple clinics or pharmacies to obtain more of a controlled substance.
When prescribing methadone, providers should check the PDMP for both methadone and other prescription opioids so that they are fully aware of other medications clients may be receiving from other places. Regular urine drug screening should be performed to make sure clients are not using other substances not obtained by prescription and that they are testing positive for methadone, meaning they are genuinely taking it if administration is not observed (2).
At the beginning of treatment, methadone is given in the office under a nurse's supervision, and then clients are monitored for adverse effects. Some take-home doses (up to 7 in the first two weeks) may be arranged for weekends or during travel, but this possibility is limited during the first few weeks of treatment. As treatment progresses and compliance is demonstrated, clients may self-administer more doses at home (up to 28 doses per month) and go longer between visits to the clinic. The total length of treatment varies but is often 1-2 years and can even be indefinite (7).
There are methadone clinics that work entirely in the scope of addiction management, but primary care providers may prescribe methadone as well. Prescribers must have an active DEA license and comply with state-based controlled substance regulations (2).
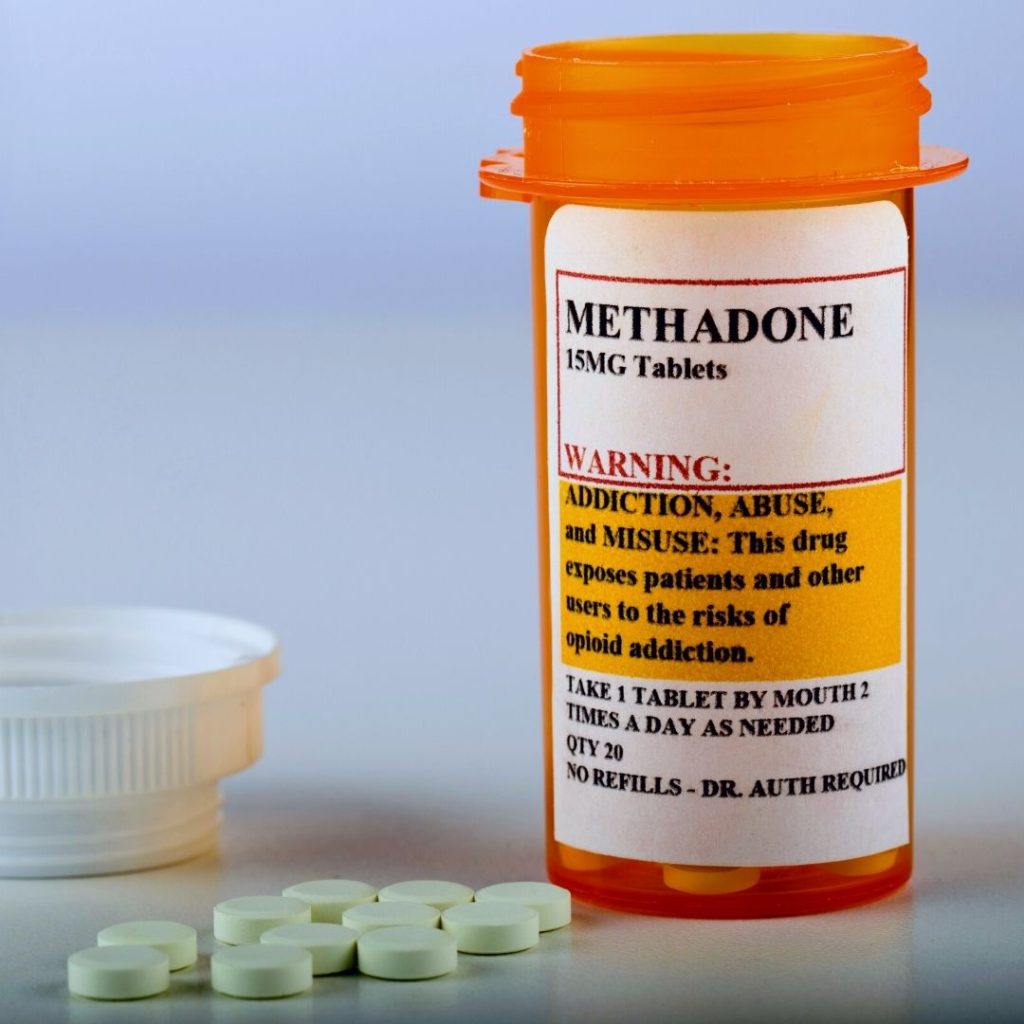

Self Quiz
Ask yourself...
- Why do you think methadone is a Schedule II Controlled Substance while buprenorphine is only a Schedule III?
- What are the benefits of checking the serum level of methadone?
- What might the clinical presentation be for someone overdosing on methadone?
- Have you ever used the PDMP database before? What are the benefits of accessing this database?
Naltrexone
Mechanism of Action and Metabolism
Naltrexone has been in use since the 1960s and is an opioid antagonist. It competes primarily with the mu-receptor but also serves as an antagonist at the kappa and delta receptors. As an antagonist, it competes with agonists such as opioids and alcohol and blocks the effects of agonists at those sites.
- Prevents euphoria.
- Prevents intoxication.
- Reduces tolerance (6)
Naltrexone also acts on the hypothalamic-pituitary-adrenal axis, modifying it to reduce cravings and suppress alcohol consumption.
It is FDA-approved for use in clinical practice for the treatment of:
- Alcohol use disorder
- Opioid use disorder (prescription and non)
Naltrexone is absorbed orally and undergoes extensive metabolism via the first-pass effect. However, this does not affect its potency as naltrexone's active metabolite, 6β-naltrexone, acts as a potent opioid antagonist. The medication's half-life is around 4 hours but can last up to 24 hours. If administered parenterally, it bypasses the first pass and is even longer acting, with a half-life of 5-10 days. Naltrexone is excreted by the kidneys (6).
Available Forms
Naltrexone is available in an oral tablet and IM injection. Available preparations include:
- Generic naltrexone tablets
- Revia (oral tablet)
- Depade (oral tablet)
- Vivitrol (solution for IM injection, extended-release) (6)
Dosing and Monitoring
Since naltrexone will compete for and block all opioid receptor sites, the risk for withdrawal symptoms is high, and clients must stop the use of alcohol or opioids for 7-10 days before beginning treatment to lessen the risk of withdrawal symptoms. A naltrexone challenge is recommended at the start of therapy.
This consists of administering small amounts of naltrexone subcutaneously or via IV and monitoring the client and their vital signs for signs of withdrawal, such as:
- Nausea
- Vomiting
- Diaphoresis
- BP changes
- Tachycardia
- Rhinorrhea
- Agitation
- Tremors
- Abdominal pain
- Pupillary dilation (6)
If a client fails the naltrexone challenge and has not been long enough since their last use of alcohol or opioids, the naltrexone initiation should be delayed, and the test should be repeated in 24 hours. If clients tolerate the naltrexone test and the negative result, they may begin naltrexone treatment (6).
For oral tablets, dosing usually starts at 25 mg for the first dose. Clients are observed for withdrawal symptoms and side effects; an additional 25 mg is given 1 hour later. After that, clients take 50 mg per day. Clients may continue with 50mg daily or take 100 mg every other day or 150 mg every 3rd day (6).
Alternatively, naltrexone may be given via IM injection for more extended action, improving compliance and reducing relapse. Particularly for alcohol or heroin dependence, data indicates that the IM route has much higher success rates than the oral route. If a client receives the IM injection, 380 mg is given to the gluteal muscle every four weeks (6).
Side Effects and Contraindications
Most common side effects of naltrexone include:
- GI irritation
- Diarrhea
- Abdominal cramps
- Nausea
- Vomiting
- Hypertension
- Headache
- Anxiety
- Low energy
- Joint or muscle pain
- Nervousness
- Sleep disruption
Less commonly, clients report:
- Loss of appetite
- Constipation
- Dizziness
- Irritability
- Depression
- Rash
- Chills (6)
Caution should be used for clients with liver function issues and renal impairment. It is Category C for use during pregnancy, and the risks versus benefits of use in pregnancy must be carefully considered. It also crosses into breast milk and must be considered carefully.
There is limited data about the overdose of naltrexone, and there may be very few symptoms if an overdose occurs. Clients should be monitored for signs of liver dysfunction, seizures, depression, and suicidal ideations. No antidote for naltrexone is currently available.
Naltrexone is contraindicated for clients who failed a naltrexone challenge, test positive for opioids or alcohol on drug screening, have a history of seizures, or have experienced a past hypersensitivity reaction to naltrexone.
Clients may switch from buprenorphine or methadone to naltrexone at some point in treatment. Both medications are agonists at the opioid receptor sites, so changing to naltrexone (an antagonist) may increase the risk of withdrawal symptoms for the first two weeks of treatment (6).
Considerations for Prescribers
Because naltrexone does not cause any euphoria or "high," the abuse potential is non-existent. It is not a controlled substance and can be prescribed by any clinician with prescriptive authority. However, its use is typically only by those who work in mental health or addiction medicine. Clients can take the medication at home or go to the clinic for IM injections.
Many considerations for naltrexone use center around monitoring for side effects and treatment compliance. Baseline and periodic drug screening and liver function tests are prudent. Clients' support persons should be educated on compliance and signs of relapse. The IM formulation should be considered for those with poor compliance or most at risk for relapse (6).

Self Quiz
Ask yourself...
- Why might a client benefit from the IM formulation of naltrexone instead of the oral preparation?
- Why might compliance with an opioid antagonist be more complex than an opioid agonist like methadone or buprenorphine?
- How do side effects differ between naltrexone and the agonist medications like methadone?
- What does it mean if a client fails a "naltrexone challenge," and how does this delay their care?
Nursing Considerations
Nurses will encounter clients with addiction and even those receiving MAT in a variety of settings, including:
- Outpatient clinics for routine care of any health issues
- ED admission for acute problems not related to addiction.
- Inpatient hospitalization related to other health problems.
- Outpatient setting for participation in MAT or addiction management.
- ED admission for acute problems related to substance abuse or toxicity of MAT medication.
- Inpatient mental health admission for mental health and addiction issues
Regardless of the setting and if the client is being seen for an addiction issue or something else, it is crucial for nurses to be familiar with MAT medications and how they work to provide safe and competent care. Nurses may need to:
- Administer medication.
- Monitor lab results.
- Observe for side effects, toxicity, or withdrawal symptoms.
- Coordinate care within a multidisciplinary team
- Communicate with therapeutic and nonjudgmental techniques.


Self Quiz
Ask yourself...
- Have you ever cared for a client in a non-addiction setting who had a MAT medication on their drug list?
- Did you have any biases or preconceived ideas about what this medication meant?
- Is there anything you have learned throughout this course that will change your care the next time you encounter a client receiving MAT?
Case Study
Justin is a 32-year-old male who presents to the ED with nausea, lethargy, and confusion worsening over the last 24 hours. Upon exam, the nurse notes diaphoresis, slurred speech, and pinpoint pupils. His vitals are RR 10, HR 54, BP 82/58, SPO2 97%, Temp 99.0.
He reports taking Wellbutrin 150mg daily for depression and smoking cessation, methadone 100mg daily for history of oxycodone abuse, and was started on ciprofloxacin 250mg BID for a UTI 2 days ago at urgent care.
His labs are significant for a WBC of 15,000 but otherwise regular. He tests positive for methadone, which is expected, but not for other substances. He reports being compliant with MAT and avoiding opioid use for nine months.
It is determined that Justin is experiencing methadone toxicity due to the slowed metabolism of the drug from the combination of methadone and ciprofloxacin. He is given naloxone in the ED, and within an hour, his symptoms have improved significantly, and his vital signs are typical. His antibiotic is switched to cefdinir, and he is discharged home in stable condition with instructions to follow up with his PCP within 1-2 days.

Self Quiz
Ask yourself...
- Given Justin's presentation, how could you differentiate between methadone toxicity and relapse?
- How might Justin's condition have progressed if he had not sought emergency care?
- How would Justin's case have been different if he had not tested positive for methadone?
- In what ways could Justin's care before his ED visit have been improved to avoid this complication?
Conclusion
Substance use disorders are a long-standing and dangerous pathology experienced by millions of people each year. At the same time, the stigma of seeking help for such disorders has been eroding in recent years; there has also been a renewed push by the federal government to address the issue in evidence-based and meaningful ways, with access to effective treatment being at the top of the priority list.
Addiction treatment programs utilizing MAT will likely become much more popular in the coming years, and nurses will be on the front lines of this therapy. For nurses to provide competent and comprehensive care to this client population, up-to-date and accurate knowledge is necessary.
Following a DNR: An Ethical Dilemma in Nursing
Introduction
End-of-life issues are often full of emotion and difficult to deal with for all involved. Do-not-resuscitate (DNR) orders can present many moral and ethical dilemmas in nursing. It takes the entire healthcare team, including the patient and their family, to ensure that all final wishes for the patient are followed. In order to understand this ethical dilemma in nursing, we must first define what ethical dilemmas are and what a DNR order is.
Nursing Ethics/Ethical Dilemma
Ethics are a system of moral principles or rules of conduct recognized by a particular group. However, the American Nurses Association (ANA) has developed its own code of ethics. The ANA Code of Ethics with Interpretive Statements includes nine provisions that direct a nurse’s moral and ethical practice. It reads:
Provision 1: The nurse practices with compassion and respect for the inherent dignity, worth, and unique attributes of every person.
Provision 2: The nurse's primary commitment is to the patient, whether an individual, family, group, community, or population.
Provision 3: The nurse promotes, advocates for, and protects the rights, health, and safety of the patient.
Provision 4: The nurse has authority, accountability, and responsibility for nursing practice; makes decisions; and takes action consistent with the obligation to provide optimal patient care.
Provision 5: The nurse owes the same duties to self as to others, including the responsibility to promote health and safety, preserve wholeness of character and integrity, maintain competence, and continue personal and professional growth.
Provision 6: The nurse, through individual and collective effort, establishes, maintains, and improves the ethical environment of the work setting and conditions of employment that are conducive to safe, quality health care.
Provision 7: The nurse, in all roles and settings, advances the profession through research and scholarly inquiry, professional standards development, and the generation of both nursing and health policy.
Provision 8: The nurse collaborates with other health professionals and the public to protect human rights, promote health diplomacy, and reduce health disparities.
Provision 9: The profession of nursing, collectively through its professional organizations, must articulate nursing values, maintain the integrity of the profession, and integrate principles of social justice into nursing and health policy.
An ethical dilemma in nursing arises when decisions are made that go against the ANA Code of Ethics with Interpretive Statements.
It is important to note that the nurse's main duty is to be an advocate for their patient, meaning that all actions should be in the patient’s best interest. Adhering to this principle will ensure a clear moral path where ethical dilemmas in nursing can be avoided (1).

Self Quiz
Ask yourself...
- What are ethics?
- How many provisions are in the ANA Code of Ethics?
- State 3 provisions of the ANA Code of Ethics.
- What is an ethical dilemma?
DNR
A DNR order is a situation where, should the patient's health deteriorate and progress to cardiac arrest, the healthcare team will not provide cardiopulmonary resuscitation (CPR). The physician usually gives this order after consulting with the patient and family. Should the patient be unable to make decisions about their health, their designated power of attorney (POA) for healthcare decisions will be able to make that determination.
The DNR order is usually reserved for patients who are gravely or terminally ill and have a strong possibility of dying during their stay at the hospital. Once the DNR is ordered, it will stay in effect until the patient passes, leaves the hospital, or rescinds the order (3, 5).

Self Quiz
Ask yourself...
- What does DNR stand for?
- What type of patient would typically have a DNR order?
- How long is the DNR order in effect?
- What is CPR?
Possible Ethical Dilemmas with a DNR
There are different situations where a DNR order could pose an ethical dilemma for the nurse. All of these examples involve the nurse's feelings, beliefs, or morals contrasting against the DNR order or the circumstances leading to the DNR order.
- Operating Room/Procedural Suite: In many facilities and healthcare systems, when a patient is undergoing surgical intervention, the DNR is suspended while the patient is in the surgical suite. The consent form for the procedure will indicate that the DNR order will be suspended, and the patient/family must agree to this to proceed with the operation.
- Should something happen during the procedure/operation, is it right to suspend the patient's DNR wishes? The hospital has metrics, including operating room mortality, that they must keep low. Suspending the DNR gives the facility a better opportunity to meet these metrics. These metric results can be viewed and compared from hospital to hospital.
- Further, a DNR order can create confusion among the surgical team. Each member may have a different idea of precisely what the DNR means. Some team members may not even believe surgery should occur, given that the patient has a DNR order. In particular, the anesthesiologist may face an ethical dilemma when providing anesthesia to a patient with a DNR order. The anesthesia can cause cardiopulmonary arrest; if the anesthesia causes such, does treating the anesthesia-induced arrest go against the DNR order (4)?
- Suffering: Another dilemma regarding a DNR order is the idea of patient suffering. It is tough to quantify and qualify suffering. That being said, one of the nurse's prime responsibilities regarding their patients is to relieve suffering. No nurse anywhere likes to see their patient in pain and suffering. In their attempts to help reduce that suffering, the nurse may push the patient or the patient's family into considering a DNR order even when it may be inappropriate given the patient's diagnosis and prognosis.
- This thought could extend to the nurse asking the physician to consult hospice services. It must be clear that not every patient who is suffering should have a DNR order; unfortunately, pain and suffering sometimes go hand-in-hand with recovery.
- There are also many different types of suffering: physical, emotional, mental, spiritual, etc. Is it right to initiate a DNR order based on these types of suffering alone? Who can say what suffering is, especially when it can't be seen (6)?
- Religion: Another factor that can impact the ethics of a DNR is the religious beliefs of the patient/family as well as the beliefs of the nurse.
- Some religions do not condone the idea of a DNR. This may stem from an erroneous understanding that the DNR is somehow assisting or facilitating death. This is not the case. A DNR means that no heroic measures will be taken should the patient stop breathing or should their heart stop beating.
- Yet, the stigma remains. There have also been cases where, when the prognosis was poor and the patient had deteriorated, a DNR was put into place. Later, the family reversed the DNR as they believed a miracle could happen and wanted to give time for their deity to move. Now, the family may be at odds with the healthcare team. One knows that God can perform a miracle and save the patient, and the other knows that the end is inevitable.
- The nurse's religious beliefs can also play a part. The nurse may believe that the DNR is premature and that the patient should still fight for life. Maybe the patient is a young one or someone who seems to have much to live for and should not give up. This nurse may find it hard just to let the patient go and could call a Code Blue despite the DNR order (2, 6).
- Capacity: The nurse needs to assess the patient's ability to make decisions for themselves, especially when a patient's faculties may come into question at the end of life. The decision for DNR is not one to be taken lightly; it is a life-or-death decision. The patient must understand what it means to be DNR, how it will affect the care plan, and what it means for their family and loved ones. Allowing the patient to make such a decision based on their condition, though their faculties may be compromised, could become an ethical dilemma (6).
- Effects on treatment: It must be noted that DNR means do not renew, not do not treat. That being said, the perception of the care that should be provided to a patient with a DNR order decreases dramatically. The idea that a DNR patient should have any procedure or operation is often scoffed at. This is especially evident when it comes to procedures meant to provide comfort but also have life-prolonging results.
- Procedures such as placing a gastrostomy tube to deliver parenteral nutrition may be needed for patient comfort and health but could be perceived as contrary to the DNR order. Also, nurses are far less likely to call a "rapid response" on a DNR patient if their condition begins to deteriorate; the nurse may not even call the physician until the patient passes because the DNR was in place. The patient should be treated as any other patient until the parameters of the DNR order are met. Nurses need to be aware of their own biases regarding DNR. Treatment should not be withheld or altered because of the DNR (2, 5, 6).

Self Quiz
Ask yourself...
- Name two possible types of ethical dilemmas concerning DNR.
- Why is a DNR order suspended when a patient undergoes surgery?
- How may a DNR order confuse the surgical team?
- Should all suffering patients have a DNR order?
How to Avoid Ethical Dilemmas with DNR
All parties agree that the best way to avoid any ethical dilemma regarding a DNR order is to have clear communication. Patients need to communicate their wishes to all their immediate family members. This will keep everyone on the same page and inform them about the patient's desires. Their end-of-life wishes need to be clear and without any confusion. In this way, the patient's wishes can be met despite what the family may believe.
It would be well advised for patients approaching the end of life to appoint a medical power of attorney who will ensure that all their expectations are followed. The decision for a DNR order must also be communicated to the healthcare team. It may not be enough to speak about the desire for the DNR, but also the expectations of their healthcare needs leading up to death as well.
The patient, their family, and the healthcare team must all understand what DNR means when it comes into play and how it will impact their care. Everyone involved in the patient's care must agree with the care plan, including the DNR. The patient and family should be educated about their diagnosis, disease process, prognosis, and treatments. In other words, a decision to have a DNR order must be made in advance.
If nurses are unable to reconcile the DNR decision within themselves even after discussing the issues with the healthcare team, they may need to step away from the situation (5).
Managing Conflict in a Nurse Leader Role
Conflict
Conflict is a natural occurrence within human interactions. It is an internal discord resulting from actual or perceived differences in ideas, objectives, values, goals, preferences, attitudes, expectations, beliefs, or feelings between two or more parties (8). Conflict can occur between any healthcare team member, including nurses, nursing students, managers or nurse leaders, physicians, patients, and family members.
Evoking feelings of hostility, anxiety, and stress can be highly disruptive to a unit or department's functioning. Another definition includes a disagreement through which the parties involved perceive a threat to their needs, interests, or concerns. In the workplace, conflict often involves personal agendas, insights, or goals (11).
Conflict is often seen as unfavorable, but if managed correctly, it can be positive and lead to personal and organizational growth. Conflict can promote team-building skills, critical thinking, new ideas, and alternative resolutions (11). If conflict is not managed effectively, it can hinder a healthcare team from providing quality patient care and can escalate into violence and abuse (16). Because of this, nurse leaders need to be aware of how conflict can escalate and be prepared to prevent or manage it in the workplace (8).
Stages
Once you have accepted that conflict is inevitable, you can learn how to deal with it within the nursing leader role. Awareness of these five stages will help you identify conflict and intervene before it escalates. Conflict within organizations often follows five different stages.
Stage 1: Potential opposition or incompatibility – Organizations themselves can cause conflict due to environmental factors. Factors within the organization include:
- A lack of communication or misunderstandings can cause communication difficulties.
- Leadership styles, role hierarchy, or reward systems
- Personal variables such as differences in values or beliefs
Stage 2: Condition and personalization- Those within the organization become aware of the environmental factors causing conflict and perceive them as unpleasant. Negative emotions develop, such as anger, frustration, or anger, and are aggressively directed at management or supervisors.
Stage 3: Intentions—Once one or both parties involved have identified conflict, it needs to be decided how the conflict should be managed. Conflict is often handled using five conflict management strategies: accommodating, avoiding, collaborating, compromising, and forcing.
Stage 4: Behavior- conflict has become visible to others, and managers or human resources intervene.
Stage 5: Outcomes—Conflict has now resulted in positive consequences that can lead to improved outcomes. Alternatively, it can remain unresolved and divide individuals, creating cliques or "sides," which leads to gossip, hostility, chronic complaining, competing for power, and decreased productivity.

Self Quiz
Ask yourself...
- When fulfilling a nurse leader role, why should you be responsible for conflict management?
- Think about a work conflict. At what stage was it managed?
- List three stages of a conflict?
- Give one definition of conflict.
Types of Conflict
The first step in managing conflict is to identify the type of conflict. Conflict can be categorized into three different types that nurse leaders will encounter. Understanding what type will help dictate how you will respond and enhance your conflict resolution skills. Healthcare and nursing present a unique environment, which can lead to different kinds of conflict. We all bring our values, ideas, thoughts, and education to this work environment. Conflict can occur in other types: interpersonal, organizational, intrapersonal, intra-group, or inter-group. Let’s break these down.
Interpersonal conflict (3)
- Most common type of conflict
- This can occur between fellow nurses, nursing students, patients, or colleagues.
- This occurs when two people can not agree on a topic; in other words, they can not see eye to eye.
This can often happen due to the following:
- Shortage of information
- Environmental stressors
- Differences in morals or opinions
- This can lead to social distancing, dominance, miscommunication, stigmatization, prejudice, stereotyping, coercion, and bullying.
- Obstructs professional interactions between healthcare teams, which weakens overall collaboration
- Example: When a team member feels another team member is not pulling their weight, or they disagree with another nurse’s assessment or a physician’s recommendation
Intrapersonal conflict (3)
- Internal disagreement with your thoughts, emotions, or values
- Can arise from ethical concerns, work-life imbalance, or uncertainty about role
- It can be the most challenging to solve
- This can often result in burnout
- Example: Nurse is overwhelmed with nursing tasks while trying to find the time for bathroom and meal breaks or if they are at work and their child is at home sick
Organizational (3)
- Disagreement occurs within large organizations such as hospitals. When policies, procedures, or communication patterns. differ
- Example: How are hospital funds distributed? Does the money go to the lab for new equipment or the emergency room for new stretchers? Conflict arises when one group believes their need is more important than the other.
Inter-group (3)
- Conflict exists between members of different organizational groups that have different values, beliefs, or goals. It could be team members who feel like their needs are unheard of or feel undervalued by administration or management, or it can occur between different units or departments. Conflict such as this can decrease productivity and interrupt quality patient care throughout the organization.
Intra-group (3)
- Conflict occurs between members of the same team (unit or department). Teamwork and a sense of belonging are essential for running a unit smoothly. Differences and disagreements can cause tension for everyone, even if they are not directly involved in the conflict. If this type of conflict is left untreated, it will divide the unit/department into groups, creating cliques or "sides," which leads to gossip, hostility, chronic complaining, competing for power, and decreased productivity.

Self Quiz
Ask yourself...
- Can you identify the different types of conflict?
- What signs would you see when experiencing an interpersonal conflict?
- Have you experienced any of the types of conflict listed?
- How would you explain the differences between inter-group and intra-group to a fellow nurse manager?
Generational Conflict (4)
Generational Differences
The generational conflict has grown within organizations due to having more generations than ever working together, especially in healthcare. Unprecedented, the nursing workforce spans five generations, each with distinct characteristics and work ethics. The various generations are described as follows:
- Baby Boomers: Born between 1947 – 1964. Characteristics include a belief that workers need to pay their dues. Often, the generation includes workaholics with traditional learning styles; rewards are expected for hard work (3).
- Generation X: Born between 1965 – 1977. Characteristics include independence, self-reliance, and skepticism of authority. This generation tends to work better with flexibility (3).
- Millennials (or Generation Y): These people were born between 1978 and 1991. Their characteristics include being goal-oriented, technological, entrepreneurial, and needing feedback. This generation wants to be coached and mentored (3).
- Generation Z: This generation was born after 1991. Although we do not know much about this generation yet, some of its characteristics include tech-savviness, self-starting, and pragmaticity (3).
Most of you know the phrase, "nurses eat their young." This is not a new concept, and despite the valiant efforts of organizations, this type of conflict has persisted. Usually, horizontal, nurse-to-nurse bullying with the older generations makes the new generations feel unwelcome, incapable, and unwilling to help when asked (4). However, it is essential to note that the opposite is also a workplace issue. Younger, newer nurses are perceived as faster, able to utilize technology at lightning speed, and can cope better with variable shifts than older nurses. This gives the newer nurse a perceived power gradient over the older nurse and can be used as a form of bullying (4).
Types of Generational Conflict (4)
As previously discussed, each generation has its own defining characteristics, and conflict can arise when they are forced to work together. Each generation will have a different outlook on how work should be done, and with that comes different expectations. Let’s take a look at intergenerational conflicts and the three major differences among generations within organizations.
Behavior-based conflict
- It arises when the other's behavior due to generational differences conflicts with their own and comes from how each generation communicates. They often do not communicate with one another, which causes conflict. Examples of communication styles between the generations:
- Baby Boomers prefer face-to-face
- Gen X prefers using what they believe is most efficient
- Gen Y prefers instant messaging, social media, or emails and is often considered poor communicators
- Gen Z and Millennials prefer text messaging and instant messaging
These differences in communication styles lead to conflicts of communication and collaboration between different generations. This lack of communication can cause team members to work individually instead of collaboratively. It can also lead to work-value conflicts, intrapersonal conflicts, and other conflicts. The success of the organization or the team depends on managing these different types of communication styles.
Another behavior-based conflict is the difference in each generation's expectation of feedback. Examples of feedback expectations per generation:
- Baby Boomers like feedback but not constantly
- Gen Y and Z require constant feedback
Conflicts arise based on how different generations view and expect feedback; it can cause frustration among team members and potentially affect teamwork and patient care.
Value-based conflict
The perception is that each generation has different values (intrinsic or extrinsic) and what motivates them. Conflicts can arise if team members are not motivated with certain methods.
- Baby Boomers believe in hard work and are motivated extrinsically (promotions, titles, corner offices, reserved parking)
- Gen Xs are distrustful of organizations and are intrinsically motivated by work independence and achieving work-life balance.
- Gen Y is motivated both extrinsically and intrinsically. They value personal achievement and work-life balance and place high importance on technology's ability to make work faster and easier.
- Gen Z is intrinsically motivated and values financial stability. They understand the importance of work but will not sacrifice their lives for work success.
Identity-based conflict
This type of conflict arises when there are differences between how generations see their own identity and the identities of other generations. It comes from wanting to belong to a group or comparing themselves to different groups. Conflicts arise when generations make comparisons to one another. This is often where hostile generalizations and stereotypes that create stigma against a group come from.

Self Quiz
Ask yourself...
- Identify which generations you currently work with.
- Identify your generation. What characteristics do you possess?
- Have you been bullied? How did it make you feel?
- Did you report being bullied? Was it handled?
- Think about the last conflict you had with a coworker. How did you handle the conflict?
- Can you identify generational conflicts you have witnessed or been involved in?
Examples of Nursing Conflicts (1)
- Staff conflicts regarding workloads, staffing ratios, and shift preferences
- Interdisciplinary disagreements about treatment plans, responsibilities, or the current decision-making process
- Ethical disputes on topics such as:
- End-of-life care
- Patient autonomy
- Organizational policies
- Poor communication resulting in misunderstandings or lack of information
- Role conflicts resulting from overlapping roles or uncertainty in responsibilities
- Resource conflicts due to limited supplies, equipment, or budget allocations
Leadership
A person’s conflict management skills are only one component of effectively managing conflict in the leadership role; they help raise future managers' emotional intelligence. Leadership skills and style are likely to affect the outcomes of conflict, while the relationship between leadership styles and conflict management (8)
Qualities
Leadership, by definition, is when one group or person sets the direction for others and helps them to achieve their goals (11). Leadership is a process that requires many different qualities; this should begin with relational intelligence. Relational intelligence revolves around the idea of how you positively navigate through relationships with others. For one fulfilling a nurse leader role, this skill is necessary for your team’s success (5). Although nurse leaders are looked to when there is conflict, it is not solely their responsibility to resolve it but that of all team members (5).
Other qualities needed include integrity, critical thinking, communication, dedication, self-awareness, and professionalism. There is not an adequate or inadequate strategy to deal with conflict. However, early detection of conflict and adoption of the most effective conflict resolution behavior is essential in organizations as soon as possible (6). How these qualities are learned is an important issue. Many nurse leaders need more training to prepare them for the responsibility of conflict resolution. However, unresolved or unmanaged conflict may end with work disruption, poor performance, tardiness, absenteeism, low staff morale, low productivity, increased psychological distress, and burnout (6). It can affect the productivity of the organization.
Another essential quality to develop at all levels of nursing, especially for those in a nurse leader role, is communication skills, including both how the message is sent and how the message is received. Listening skills are a vital aspect of nursing, and often, we move quickly and think about an answer before the person finishes speaking; misunderstandings are easy to cause but only sometimes so easy to fix.
For nurse leaders, it is essential to recognize and manage conflict. Barriers often exist, including time constraints, fear of retaliation, and fear of exclusion. Of course, no one wants conflict, which sometimes results in an attempt to soothe and prematurely find a solution. Those in the nurse leader role must learn to positively engage in conflict resolution and stay committed to promoting collaboration and effective care. Being successful includes dialogue, coaching, the identification of potential conflict, education, and training.
Styles
Leadership styles are management thoughts and behaviors related to your personality, communication preferences, strengths, weaknesses, and values (11). Styles can develop over time or be influenced by the organization. To prevent or limit conflict, leaders should implement a professional code of conduct, ground rules, and discipline (11). Leadership styles in healthcare are found to be strongly tied to quality care, patient outcomes, mortality rates, injuries, patient satisfaction, and pain (11). Leadership styles that are more successful and effective include collaboration, multifaceted, and dynamic. Believe it or not, leadership styles play a role in conflict resolution and team dynamics. The most common styles leadership styles are (11):
- Servant Leader and Lean Leader: This style is team-oriented and involves openness in conversations and management. It ensures others are a priority by focusing on their needs and growth and putting them first. This style improves processes, eliminates waste, and promotes high-quality, cost-effective care.
- Authoritarian Leader: This leader leads via dictation and controls all team actions and decision-making. All decisions are based on the leader's ideas, judgments, and personal beliefs, and the leader does not consider or consult team members. The leader and little input from team members involve a lot of enforcement.
- Transformational Leader: Transformational leaders assist the team in aligning their beliefs and values with those of the organization. This leader fosters trust, relationship building, and sharing of ideas and visions for the organization. The transformational style has defined goals, a clear direction, and looks at the big picture. Those in the leadership role share their vision with staff and think outside the traditional path. This has the idea that when involved in a conflict, responses will reflect the organization's greater good. The ability to manage conflict effectively is a quality seen by transformational leaders. This style is seen as being positive.
- Lassiez-Faire Leader: The laissez-faire style is casual and laid back, meaning there needs to be more leadership; they leave the decision-making to their team members. This type of leader trusts their team members to problem solve, create new projects, make and meet goals, and self-monitor. Authority is turned over to the staff; there is no feedback, oversight, direct leadership, discipline, or praise. Productivity is often low among team members, and conflict is highly likely. Generally, this style is seen as being negative because it wants to avoid conflict and is unsuccessful.
- Visionary Leader: This leader has a vision or long-term goal in mind. These leaders often have insight, imagination, and passion related to goals and ideas. They are constantly looking out for the team's best interests by sharing ideas and goals. They empower their team members by building a solid team dynamic capable of managing conflicts via communication in a positive way.
- Transactional Leader: Leaders with this style are focused on workflow, meaning they focus on incentives for “doing the work” in a timely and efficient manner. Components of this style include rewards and discipline based on finishing work ahead of schedule or if the work is delayed. These leaders need to plan for the future of their organization by being focused on the present. This type of leadership is harmful as it fails to promote ideas required for the rapidly changing healthcare industry.
While developing a style, it is essential to allow for the expression of multiple viewpoints and how to build better relationships (11). The absentee leader needs to do more to communicate, mentor, and plan. The incompetent leader needs more involvement in planning and faces severe moral issues. If the environment is maintained and cohesive, job satisfaction and performance can be positively impacted.
Nurse Leader Role: Conflict Strategy
As one involved in a nurse leader role, in order to effectively eliminate or manage conflict, it cannot be ignored. Engagement must be an ongoing process. To be successful, one must learn to not only engage in conflict, but remain engaged to promote collaboration and effective care coordination (11). The earlier conflict is identified, the better, as dysfunctional patterns can take place and can potentially define the culture of the department.
Common Strategies (11):
- Safe Space: It is important that staff feel they are meeting in a safe space, and that there is privacy and support.
- Coaching: Coaching can be in either group or individual settings. Coaching sessions are confidential (unless otherwise agreed upon), must follow a consistent format, and include a written summary of the session.
- Facilitation: There is usually a defined agenda. You will need someone neutral who can see past the fireworks or walls of silence and assist the group with arriving at the core problems or issues.
- Dialogue: The importance is to have a discussion that addresses the issue and clears the air; avoid saying “always” and “never.” Nursing leaders and direct care nurses need to engage in dialogues that address conflict and conflict management behavior as a first step in creating a healthy work environment (1).
- Collaborative: Collaborative works with the group problem solving together.
- Storytelling: Storytelling works with traditional stories that are told to help move from personal experiences to broader, helping to negotiate group conflict.
- Mediation: Can be formal or informal.
- Education and training: Nurses need to be educated on conflict and conflict management strategies that address and effectively resolve conflict. Learning conflict management strategies empowers nurses to resolve conflict early and influence the work environment in which they deliver patient care. "The training should not be limited to the handling of interpersonal conflicts; it should include all types of conflict commonly encountered in the healthcare setting. Additionally, individuals who have a propensity for managing conflict well should be identified and developed” (1). Education and training should also include communication skills.
"Leaders can change the climate in the workplace and promote better collaboration among workers by interrupting a group's dysfunctional behavior patterns," (12). Adding the skills of self-awareness and emotional skills helps to bring a team together. Relational ethics emphasizes the importance of mutually respectful relationships. People work to improve their awareness of how their choices and actions help shape their conversations and social interactions. The relational approach addresses conflict as it unfolds – just as a relationship evolves and unfolds over time. It incorporates the essential qualities that form the core of human relationships. It is hard to imagine an approach to conflict that excludes consideration of integrity, respect, identity, compassion, humility, shame, trust, fear, hope, pride, acceptance. Love, joy, and other human dynamics are at the heart of most conflicts (12).
Emotional and social intelligence are defined as "skills that enable an individual to understand the impact of emotions on behavior and thinking, to regulate emotions and behavior, to understand the importance of emotions in others, and to understand social interactions and engage in adaptive ways with others in social situations," (12).

Self Quiz
Ask yourself...
- Define two traits of Relational Intelligence. What is your strongest trait?
- List three traits of a leader.
- Identify the successful styles of leadership.
- What type of leadership does your work unit have?
Regulatory Issues and Conflict
In 2008, The Joint Commission released a sentinel event alert addressing disruptive behaviors and recommended how organizations should address their relational issues and conflicts. The defined behaviors recognized more physician-to-nurse issues but placed all disruptive behavior on its radar. Identified behaviors included verbal outbursts, physical threats, uncooperative attitudes, and cooperation refusal (i.e., not taking pages or answering calls). Other behaviors include condescending language and refusing to complete tasks (9).
Disruptive behaviors often go unreported and, therefore, unaddressed for several reasons. Whether that is fear of retaliation, the stigma associated with "blowing the whistle" on a colleague, or a general reluctance to confront an intimidator, it all contributes to underreporting intimidating and disruptive behavior (9).
Part of the sentinel event has given guidance on what defines behaviors and required resolutions. Organizations are needed within this mandate to develop a specific ‘Code of Conduct’ that outlines acceptable and disruptive behaviors and the processes managing these behaviors. Additionally, organizations should establish policies and procedures that clearly state zero tolerance and provide training and education for physicians.
Regarding nurse-to-nurse (horizontal) disruptive behavior, the American Nurses Association (ANA) Code of Ethics for Nurses includes interpretive statements outlining that nurses are required to "create an ethical environment and culture of civility and kindness, treating colleagues, coworkers, employees, students, and others with dignity and respect" (10).
Similarly, nurses must be afforded the same respect and dignity as others; thus, the nursing profession should no longer tolerate violence from any source. As someone fulfilling a nurse leader role, you must refer to the ANA Code of Ethics for Nurses when managing conflict within the workplace.
Negative Behaviors
Familiar sources of conflict can include passive-aggressive communication, clique behaviors, lateral or vertical aggression, and other types of incivility. These sources can cause unprofessionalism and disruptive actions that can compromise patient safety, increase turnover and absenteeism, and reduce the overall joy of work (7). Let's look at the following negative behaviors often seen in conflict and how to deal with them potentially (7).
- Defensiveness (7)
- It is human nature to deflect when we hear something negative about ourselves, our interests, or our friends. Critical conversations will be redirected to focus on someone else's flaws instead.
- Redirect behavior and state facts.
- Victim mentality
- Individuals feel mistreated, singled out, or held to a higher standard.
- Remind people that personal conversations are confidential and held to the same standard as everyone else.
- Restate all expectations are clear
- Passive aggressiveness
- Confront and be direct
- Encourage open communication and concerns
- Vertical aggression
- Bullying of new leadership
- Address head-on and state expectations
- Bullying
- Identify and address immediately
- Provide clear examples of actions
- The informer
- Performance information given by peers and not leadership
- Hold team members accountable
- Address behaviors expressed by other team members

Self Quiz
Ask yourself...
- Can you recall a time when you witnessed negative behaviors of conflict?
- Do you have any behaviors among team members? If so, how does it affect productivity and patient care?
- Think about how you would manage these negative behaviors.
How to Manage Conflict (1)
Nursing requires a teamwork approach to provide safe, quality care; therefore, everyone must work together to solve conflicts. How you and your team respond to conflict will vary based on the situation and the individual personalities involved. Conflict resolution is defined in various ways. Thomas and Kilmann formulated a matrix with five distinct responses (methods, modes, or styles) to conflict resolution or management. Here are five different conflict management styles (Thomas-Kilman Conflict Modes) to try within your team when resolving conflicts.
Accommodation (11)
- Using this style results in a win-lose situation. The resolution to the conflict will only benefit one person and will not satisfy all involved. This style of conflict management can cause resentment. It is a strategy often used to maintain peace by smoothing over differences. It can be appropriate to escalate the issue if it results in a severe disruption.
Collaboration (11)
- In this particular style, all team members are brought together to resolve. It allows for solving the conflict by objectively evaluating different views. This style encourages open and respectful communication, active listening, and open-mindedness. Everyone involved will have a say in what caused the conflict and how to achieve a resolution. Collaboration often leads to new ideas and creativity and is the best outcome for all team members.
Compromise (11)
- No team members will be satisfied with the solution during this management style. The resolution to the conflict will cause resentment between all individuals involved because those involved will have to sacrifice a part of their solution. It is a bargaining strategy that protects the relationship's importance and can provide a temporary solution.
Avoidance (11)
- When using this conflict management style, some or all people involved will avoid or ignore the situation. This ends up being a losing situation, and the conflict remains unresolved. It will continue to build up disputes among team members. No cooperativeness exists between the parties involved, and no one’s concerns are met. The purpose is to delay or walk away, often due to immense anger. This is considered a short-term resolution to de-escalate an agitated, non-emergent situation.
Competition (11)
- This style creates another win-lose situation. It is a short-term solution strategy to resolve the problem. It can involve aggressively resolving the conflict when one individual has more decision-making power. It will not promote a team approach to problem-solving or resolving future disputes.
Steps for Conflict Management (11)
- Set rules on how to communicate effectively and respectfully
- Ask all team members to set aside previous judgments about one another
- Encourage active listening without interruption to ensure all are heard equally
- Have everyone involved write down the problem and then state the problem out loud. This will help to provide understanding and agreement about what is causing the conflict.
- Ask everyone involved to devise a solution to the problem or conflict.
- Set time aside to discuss each solution along with the positives and negatives of each solution.
Nurse Leader Role: Conflict Strategy Resolution
As one involved in a nurse leader role, it must be addressed to eliminate or manage conflict effectively. Ignoring the conflict or problem can result in your team developing resentment towards one another, making the work environment uncomfortable (1). Rushing to fix conflicts without determining the source of the issue can often lead to other future problems (1).
You must take the time to analyze the situation and identify the source of the conflict before proceeding to resolution. Conflict resolution requires patience, active listening skills, and commitment to finding a solution that benefits both parties (1). The goal is to address conflicts within your team and promote a safe and harmonious work environment that provides quality patient care (1). Engagement must be an ongoing process. To be successful, one must learn to not only engage in conflict but also remain engaged to promote collaboration and effective care coordination (1). The earlier conflict is identified, the better, as dysfunctional patterns can take place and potentially define the department's culture. Conflict resolution in nursing requires communication, collaboration, and listening skills. Here are some tips for handling conflicts:
Common Strategies (1):
- Safe Space: Create a safe place or mutual ground for all parties involved. It is essential that staff feel they are meeting in a safe space and that there is privacy and support.
- Guidance and Coaching: Develop effective communication and conflict-resolution skills and support suggestions for productive and creative solutions. Coaching can be in either group or individual settings. Coaching sessions are confidential (unless otherwise agreed upon), must follow a consistent format, and include a written summary of the session.
- Facilitation: There is usually a defined agenda. You will need someone neutral who can see past the fireworks or walls of silence and assist the group in identifying the core problems or issues.
- Foster Open Communication: Create a supportive environment that encourages active listening and honest communication. Be sure to understand the situation entirely. It is essential to have a discussion that addresses the issue and clears the air; avoid saying “always” and “never.” Nursing leaders and direct care nurses must engage in dialogues that address conflict and conflict management behavior as a first step in creating a healthy work environment.
- Collaborative: Collaborative works with the group problem-solving together.
- Storytelling: Storytelling uses traditional stories to help people move from personal experiences to broader ones and negotiate group conflict.
- Mediation: This can be formal or informal.
- Education and training: Nurses need to be educated on conflict and conflict management strategies that address and effectively resolve conflict. Providing team members with strategies to resolve disputes early before impacting patient care is essential. Learning conflict management strategies empowers nurses to resolve conflict early and influences the work environment in which they deliver patient care. Training should include all strategies one would encounter in the healthcare setting, not just those limited to coworkers. Education and training should also include communication skills.
- Mediate and negotiate: Approach the conflict objectively and seek additional perspectives from human resources or another nurse leader if necessary. Facilitate constructive dialogue to reach a mutually agreeable solution.
- Identify underlying issues: Identifying the conflict's root cause is crucial. Work on finding solutions that aren’t quick fixes and address the core problem. This is also an opportunity for growth and development, including developing policies and procedures to help identify future conflicts.
- Encourage empathy: Ask questions that promote different perspectives and creative solutions. Identify and discuss underlying interests that can often interfere with views.
- Seek a compromise: Find common ground and work together toward a mutual agreement. Stress the importance of collaboration.
- Encourage professional behavior: Rule out and quickly address bullying and rude, discourteous, or disrespectful behaviors. Identify, admit, and apologize if you have contributed to the conflict.
- Follow established policies and procedures: Be consistent with standards and potential consequences while handling conflicts fairly and openly.
- Document the issue and provide follow-up: Document the situation, steps taken, and the resolution. Continue to monitor to ensure the problem is resolved and does not reoccur
"Leaders can change the climate in the workplace and promote better collaboration among workers by interrupting a group's dysfunctional behavior patterns (12)." Adding self-awareness and emotional skills helps bring a team together. Relational ethics emphasizes the importance of mutually respectful relationships. People work to improve their awareness of how their choices and actions help shape their conversations and social interactions. The relational approach addresses conflict as it unfolds – just as a relationship evolves and unfolds over time. It incorporates the essential qualities that form the core of human relationships. It is hard to imagine an approach to conflict that excludes consideration of integrity, respect, identity, compassion, humility, shame, trust, fear, hope, pride, and acceptance. Love, joy, and other human dynamics are at the heart of most conflicts (12).
Emotional and social intelligence is defined as "skills that enable an individual to understand the impact of emotions on behavior and thinking, to regulate emotions and behavior, to understand the importance of emotions in others, and to understand social interactions and engage in adaptive ways with others in social situations" (12).
References + Disclaimer
California Schedule II Controlled Substances and Risk of Addiction
- Azadfard , M., Huecker , M., & Leaming , J. (2023, April 29). Opioid addiction – statpearls – NCBI bookshelf. StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK448203/
- Campbell, J. N. (1996, March). APS 1995 Presidential address. APS 1995 Presidential address. In Pain Forum (Vol. 5, No. 1, pp. 85-88). .
- Chou , R., Deyo , R., Devine, B., Hansen, R., Sullivan, S., Jarvik , J., Blazina , I., Dana, T., Bougatsos, C., & Turner, J. (2014, September). The effectiveness and risks of long-term opioid treatment of chronic pain. The Effectiveness and Risks of Long-Term Opioid Treatment of Chronic Pain | Effective Health Care (EHC) Program. https://effectivehealthcare.ahrq.gov/products/chronic-pain-opioid-treatment
- Department of Health and Human Services Pain Management Best Practices Inter-Agency Task Force. (2019) Pain Management Best Practices Inter-Agency Task Force Report: Updates, Gaps, Inconsistencies, and Recommendations. Final Report. Washington, DC: Content created by Assistant Secretary for Health (ASH); 2019. https://www.hhs.gov/ash/advisory-committees/pain/reports/index.html;
- Dowell, D., Ragan, K. R., Jones, C. M., Baldwin , G. T., & Chou, R. (2022, December 1). Prescribing opioids for pain — the new CDC clinical practice guideline … New England Journal of Medicine. https://www.nejm.org/doi/full/10.1056/NEJMp2211040
- Dowell, D., Ragan, K. R., Jones, C. M., Baldwin, G. T., & Chou, R. (2022). CDC Clinical Practice Guideline for prescribing opioids for pain, United States, 2022. MMWR. Recommendations and Reports, 71(3), 1–95. https://doi.org/10.15585/mmwr.rr7103a1
- Dydyk AM, Sizemore DC, Haddad LM, Lindsay L, & Porter BR. (2023, January 29). NP safe prescribing of controlled substances while avoiding drug diversion. National Center for Biotechnology Information. https://pubmed.ncbi.nlm.nih.gov/33232099/
- Dydyk, A. M., & Conermann, T. (2023, July 23). Chronic pain – statpearls – NCBI bookshelf. StatPearls . https://www.ncbi.nlm.nih.gov/books/NBK553030/
- Federal Controlled Substance Act 21 USC 844 (1970).
- James, A., & Williams, J. (2020). Basic opioid pharmacology — an update. British Journal of Pain, 14(2), 115–121. https://doi.org/10.1177/2049463720911986
- Loeser, J. D., & Melzack, R. (1999). Pain: An overview. The Lancet, 353(9164), 1607–1609. https://doi.org/10.1016/s0140-6736(99)01311-2
- Mayo Clinic Staff. (2022, April 12). Am I vulnerable to opioid addiction?. Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/prescription-drug-abuse/in-depth/how-opioid-addiction-occurs/art-20360372?p=1
- Medical Board of California. (2023). Guideline for Prescribing Controlled Substances for Pain. Medical Board of California. July, 2023. https://www.mbc.ca.gov/Download/Publications/pain-guidelines.pdf
- Newson, G. G. (2004, December 4). Criteria for furnishing number utilization by Nurse Practitioners . State of California Department of Consumer Affairs. https://www.rn.ca.gov/pdfs/regulations/npr-i-16.pdf
- PDR Search. PDR.Net. (n.d.). https://www.pdr.net/
- Smith, H. J. (2020). Ethics, public health, and addressing the opioid crisis. AMA Journal of Ethics, 22(8). https://doi.org/10.1001/amajethics.2020.647
- Spencer, M., Miniño, A., & Warner, M. (2022). Drug overdose deaths in the United States, 2001–2021. NCHS Data Brief, (457). https://doi.org/10.15620/cdc:122556.
- Tighe, P., Buckenmaier, C. C., Boezaart, A. P., Carr, D. B., Clark, L. L., Herring, A. A., Kent, M., Mackey, S., Mariano, E. R., Polomano, R. C., & Reisfield, G. M. (2015). Acute pain medicine in the United States: A status report. Pain Medicine, 16(9), 1806–1826. https://doi.org/10.1111/pme.12760
- Trachsel, L. A., Munakomi, S., & Cascella, M. (2023, April 17). Pain theory: Treatment & management: Point of care. StatPearls. https://www.statpearls.com/point-of-care/26535
- U.S. Department of Justice Drug Enforcement Administration, Diversion Control Division. (2023, July). Controlled Substance Schedules. Controlled substance schedules. https://www.deadiversion.usdoj.gov/schedules/
Schedule II Controlled Substances and Neonatal Abstinence Syndrome
- American College of Nurse-Midwives. (2021). Scope of Practice for Certified Nurse-Midwives and Certified Midwives. https://www.midwife.org/ACNM/files/ccLibraryFiles/Filename/000000007973/SOP-Quick-Reference-2021.pdf
- American College of Obstetricians and Gynecologists. (2018). Postpartum Pain Management. Obstetrics & Gynecology, 132(1), e35-e43.
- American Nurses Association. (2019). ANA’s Principles for Pain Management. American Nurse, 51(3), 1-4.
- Bartley, E. J., & Fillingim, R. B. (2013). Sex differences in pain: A brief review of clinical and experimental findings. British Journal of Anaesthesia, 111(1), 52-58.
- California Board of Registered Nursing. (2021). Dispensing in California. https://www.rn.ca.gov/pdfs/regulations/npr-b-33.pdf
- California Code of Regulations, Title 16, Section 1746.51. https://govt.westlaw.com/calregs/Document/I98B2EF80D1E011E4B5EAF829D5A0D425?viewType=FullText&originationContext=documenttoc&transitionType=CategoryPageItem&contextData=(sc.Default)
- California Department of Public Health. (2021). Neonatal Abstinence Syndrome (NAS). https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH%20Document%20Library/MCAH/NAS_FactSheet_2016.pdf
- California State Board of Pharmacy. (2020). Schedule II Controlled Substance Prescription Requirements. https://www.pharmacy.ca.gov/licensees/schedule_ii_prescription.pdf
- Patrick, S. W., Schiff, D. M., & Committee on Substance Use and Prevention. (2016). A public health response to opioid use in pregnancy. Pediatrics, 138(5), e20161559.
- Pergolizzi, J. V., Raffa, R. B., Taylor, Jr., R., & Vacalis, S. (2012). The role and mechanism of action of mu-opioid analgesics. Clinics in Anaesthesiology, 25(4), 385-400. DOI: 10.1016/j.cpa.2012.08.002
- 11.Smith, J. A., Greer, T. M., Sheets, E. S., & Davis, M. (2019). Opioid use in pregnancy and neonatal abstinence syndrome. Journal of Obstetric, Gynecologic & Neonatal Nursing, 48(3), 297-308.
- 12.Substance Abuse and Mental Health Services Administration. (2020). Preventing Neonatal Abstinence Syndrome: A Guide for Women in Recovery. https://store.samhsa.gov/sites/default/files/SMA18-5054.pdf
- 13.Volkow, N. D., & McLellan, A. T. (2016). Opioid abuse in chronic pain—Misconceptions and mitigation strategies. New England Journal of Medicine, 374(13), 1253-1263.
- PDR Search. PDR.Net. (n.d.). https://www.pdr.net/
- Dowell, D., Ragan, K. R., Jones, C. M., Baldwin, G. T., & Chou, R. (2022). CDC Clinical Practice Guideline for prescribing opioids for pain, United States, 2022. MMWR. Recommendations and Reports, 71(3), 1–95. https://doi.org/10.15585/mmwr.rr7103a1
- U.S. Department of Justice Drug Enforcement Administration, Diversion Control Division. (2023, July). Controlled Substance Schedules. Controlled substance schedules. https://www.deadiversion.usdoj.gov/schedules/
Safe Prescribing of Opioid Drugs
- Bhatnagar, P. (2022, Deptember 12). Opioid Equivalency. StatPearls. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK535402/
- Center for Diesease and Control. (n.d.). Assessing benefits and harms of opioid therapy. Retrieved from CDC: https://www.cdc.gov/drugoverdose/pdf/assessing_benefits_harms_of_opioid_therapy-a.pdf
- Center for Disease Control. (2019, August 13). Prescribing Practices. Retrieved from CDC: https://www.cdc.gov/drugoverdose/deaths/prescription/practices.html
- Center for Disease Control. (2021, May 19). PDMPS: What States Need to Know. Retrieved from CDC Drug Overdose: https://www.cdc.gov/drugoverdose/pdmp/index.html
- Center for Disease Control. (2023, April 28). CDC’s 2022 Clinical Practice Guideline for Prescribing Opioids for Pain. (C. Mikosz, & M. Dowling, Eds.) Retrieved from CDC Training for Healthcare Professionals: https://www.cdc.gov/opioids/healthcare-professionals/training/overview.html
- Center for Medicare Advocacy. (2023, June 29). Medicare Enrollment Numbers. Retrieved from Center for Medicare Advocacy (2023): https://medicareadvocacy.org/medicare-enrollment-numbers/#:~:text=33%2C948%2C778%20are%20enrolled%20in%20Original,enrolled%20in%20Medicare%20Part%20D.
- Center for Medicare and Medicaid. (2023). EPCS Getting Started Quick Reference Guide. Retrieved from Center for Medicare and Medicaid: https://www.cms.gov/files/document/epcs-program-getting-started-quick-reference-guide.pdf
- Centers for Medicare & Medicaid Services. (2020, August 4). Medicare Program: Electronic Prescribing of Controlled Substances; Request for Information. Retrieved from Federal Register: https://www.federalregister.gov/documents/2020/08/04/2020-16897/medicare-program-electronic-prescribing-of-controlled-substances-request-for-information-rfi
- Diversion Control Division. (n.d.). Electronic Prescriptions for Controlled Substances (EPCS). Retrieved from Diversion Control Division: https://www.deadiversion.usdoj.gov/ecomm/e_rx/
- Diverson Control Division. (2023). Controlled Substance Schedules. Retrieved from US Departement of Justice Drug Enforcement Administration Diversion Control Division: https://www.deadiversion.usdoj.gov/schedules/index.html.
- Dowell, D., Ragan, K., Jones, C., & Baldwin, G. (2022, December 1). Prescribing Opioids for Pain — The New CDC Clinical Practice Guideline. The New England Journal of Medicine, 2011-2013. doi:10.1056/NEJMp2211040
- Dowell, D., Ragan, K., Jones, C., Baldwin, G., & Chou, R. (2022, November 3). CDC Clinical Practice Guideline for Prescribing Opioids for Pain. 71(No. RR-3), 1-95. United States: MMWR Recomm Rep. doi: http://dx.doi.org/10.15585/mmwr.rr7103a1
- DrFirst. (n.d.). State Mandates Driving EPCS and PDMP Utilization. Retrieved from DrFirst: https://drfirst.com/resources/regulatory-mandates/
- Food and Drug Administration. (2020, October 1). Safe Disposal of Medications. Retrieved from FDA: https://www.fda.gov/drugs/safe-disposal-medicines/disposal-unused-medicines-what-you-should-know
- Food and Drug Administration. (2023, August 9). Timeline of Selected FDA Activities and Significant Events Addressing Substance Use and Overdose Prevention. Retrieved from FDA: https://www.fda.gov/drugs/information-drug-class/timeline-selected-fda-activities-and-significant-events-addressing-substance-use-and-overdose
- Geyer, D. H. (2023, January 17). A brief history of morphine use. Retrieved from Mayo Clinic Press: https://mcpress.mayoclinic.org/opioids/history-of-morphine/#:~:text=1803%3A%20Morphine%20is%20discovered&text=After%20conducting%20several%20years%20of,because%20it%20made%20people%20sleepy.
- Li, K., Harris, K., Hadi, S., & Chow, E. (2007). What should be the optimal cut points for mild, moderate, and severe pain? Journal of Palliative Medicine, 10(6), 1338-1346. doi: 10.1089/jpm.2007.0087
- National Institute on Drug Abuse. (2023, August 7). Only 1 in 5 US adults with opioid use disorder received medications to treat it in 2021. Retrieved from National Institutes of Health: https://nida.nih.gov/news-events/news-releases/2023/08/only-1-in-5-us-adults-with-opioid-use-disorder-received-medications-to-treat-it-in-2021#:~:text=News%20Release-,Only%201%20in%205%20U.S.%20adults%20with%20opioid%20use%20disorder,to%20treat%20it%20in%
- PDMP TTAC. (2020, July 10). Mandatory Enrollment and Use of PDMPs. Retrieved from Prescrption Drug Monitoring Program Training and Technical Assistance Center: https://www.pdmpassist.org/pdf/TAG_Mandatory_Enrollment_Use_20200710.pdf
- SAMHSA. (n.d.). Talking with Your Teen About Opioids. Retrieved from Substance Abuse and Mendal Health Services Administration: https://www.samhsa.gov/sites/default/files/TTHY-Opioid-Broch-2020.pdf
- Substance Abuse and Mental Health Services Administration. (2023, June 9). Naltrexone. Retrieved from SAMHSA: https://www.samhsa.gov/medications-substance-use-disorders/medications-counseling-related-conditions/naltrexone
- Substance Abuse and Mental Health Services Administration. (2021). Medications for Opioid Use Disorder TIP 63. Retrieved from SAMHSA: https://store.samhsa.gov/sites/default/files/pep21-02-01-002.pdf
- Substance Abuse and Mental Health Services Administration. (2023, June 20). Methadone. Retrieved from SAMHSA: https://www.samhsa.gov/medications-substance-use-disorders/medications-counseling-related-conditions/methadone
- Substance Abuse and Mental Health Services Administration. (2023, June 7). Waiver Elination (MAT Act). Retrieved from SAMHSA: https://www.samhsa.gov/medications-substance-use-disorders/waiver-elimination-mat-act
- The Center for Disease Control and Prevention. (2021, January 26). Opioid Commonly Used Terms. Retrieved from CDC: https://www.cdc.gov/opioids/basics/terms.html
- UAMS Psychatric Research Institute. (2023). What is Buprenorphine. Retrieved from UAMS Psychiatric Research Institute: https://psychiatry.uams.edu/clinical- care/cast/buprenorphine/
- Zhang, K. (2021, June 7). Overview of the Opioid NDC and MME Analytical File Compiled by CDC. 1-37. United States: Division of Overdose Prevention, CDC.
Tirzepatide for Type 2 Diabetes and Weight Management
- Katie Kerwin McCrimmon, Uch. (2023, May 15). What is Mounjaro? and does it work better for weight loss than Ozempic and Wegovy?. UCHealth Today. https://www.uchealth.org/today/what-is-mounjaro-and-how-does-it-work-for-weight-loss/
- National Institutes of Health. (2023, July 28). DailyMed – mounjaro- tirzepatide injection, solution. U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d2d7da5d-ad07-4228-955f-cf7e355c8cc0
- Type 2 Diabetes Treatment to Lower A1C | Mounjaro® (tirzepatide). (2023). https://www.mounjaro.com/?utm_id=go_cmp-16966473997_adg-133042365262_ad-626584063672_kwd-1651326559888_dev-c_ext-_prd-_mca-_sig-EAIaIQobChMIxcXZqYeHgQMVhDjUAR2Lvw5CEAAYASAAEgIMkfD_BwE&utm_source=google&utm_medium=ppc&campaign=16966473997&adgroup=133042365262&ad=626584063672&utm_keyword=kwd-1651326559888&gclid=EAIaIQobChMIxcXZqYeHgQMVhDjUAR2Lvw5CEAAYASAAEgIMkfD_BwE
- GoodRX – error. (2023, July 14). https://www.goodrx.com/mounjaro/how-make-you-feel
- Chen, S. (2023, July 28). Diabetes drug Mounjaro shown to have extraordinary weight loss for people without diabetes. diaTribe. https://diatribe.org/diabetes-drug-mounjaro-shown-have-extraordinary-weight-loss-people-without-diabetes
- VA Pharmacy Benefits Management Services. (2022, August). Tirzepatide Injection Criteria for Use. https://www.va.gov/formularyadvisor/DOC_PDF/CFU_Tirzepatide_MOUNJARO_Criteria_Aug_2022.pdf.
- Allied PR. (2023 B.C.E., May 6). Mounjaro Weight Loss: Newly Launch Over The Counter Alternative Pills To Tirzepatide & Mounjaro Weight Loss. https://newsdirect.com/news/mounjaro-weight-loss-newly-launch-over-the-counter-alternative-pills-to-tirzepatide-and-mounjaro-weight-loss-300908569. Retrieved August 30, 2023, from https://newsdirect.com/news/mounjaro-weight-loss-newly-launch-over-the-counter-alternative-pills-to-tirzepatide-and-mounjaro-weight-loss-300908569
- Mounjaro (2023). Savings and support for Mounjaro. Retrieved on August 31st 2023 from https://www.mounjaro.com/savings-resources?gclid=Cj0KCQjw9MCnBhCYARIsAB1WQVWAcINPKFwiHl4SbZQiGC8E_o9wHVOkDJDDUuphW3OlsKBagVTF1rQaApLJEALw_wcB
Semaglutide and Type 2 Diabetes
- Global diabetes cases to soar from 529 million to 1.3 billion by 2050. (n.d.). The Institute for Health Metrics and Evaluation. https://www.healthdata.org/news-events/newsroom/news-releases/global-diabetes-cases-soar-529-million-13-billion-2050.
- Diabetes Research Institute Foundation. (2023, March 31). Diabetes Statistics – DRIF. DRIF. https://diabetesresearch.org/diabetes-statistics/#:~:text=37.3%20million%20people%2C%20or%2011.3,yet%20been%20diagnosed%20(2022)
- Manage blood sugar. (2021, April 28). Centers for Disease Control and Prevention. https://www.cdc.gov/diabetes/managing/manage-blood-sugar.html
- About the National Diabetes Prevention Program | CDC. (n.d.). https://www.cdc.gov/diabetes/prevention/about.htm#:~:text=The%20National%20Diabetes%20Prevention%20Program%E2%80%94or%20National%20DPP%E2%80%94was%20created,diabetes%20in%20
- Ms, M. G. R. (2023, February 16). All about Ozempic. Healthline. https://www.healthline.com/health/drugs/ozempic#_noHeaderPrefixedContent
- Professional, C. C. M. (n.d.). Ghrelin. Cleveland Clinic. https://my.clevelandclinic.org/health/body/22804-ghrelin#:~:text=Ghrelin%20and%20leptin%20are%20two,brain%20when%20you’re%20hungry
- Prelipcean, M., MD. (2020, June 22). Behind the counter: Glucagon-like peptide-1 receptor agonists for type 2 diabetes. https://www.medicalnewstoday.com/articles/glp-1-receptor-agonists-type-2-diabetes
- Mba, A. B. P. (2023, May 1). Ozempic side effects: What you should know. https://www.medicalnewstoday.com/articles/drugs-ozempic-side-effects
- Delong, C. (2023, February 11). Black box warning. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK538521/
- MNT Medical Network. (2023, February 16). Ozempic (semaglutide). https://www.medicalnewstoday.com/articles/326252
- Ozempic® vs. Other Type 2 Diabetes Treatments | Ozempic® (semaglutide) injection 0.5 mg or 1 mg. (n.d.). https://www.ozempic.com/why-ozempic/diabetes-medicines-comparison.html
- Northrop, A. (2023, August 3). Ozempic For Weight Loss: Side Effects, Risks And More. Forbes Health. https://www.forbes.com/health/body/ozempic-for-weight-loss/%20)
- Kerwin McCrimmon, UCHealth, K. (2023, April 5). Wegovy vs. Ozempic: The truth about new ‘weight-loss’ drugs. Uchealth. https://www.uchealth.org/today/wegovy-vs-ozempic-the-truth-about-new-weight-loss-drugs/#:~:text=If%20you%20take%20Wegovy%20or,to%20keep%20benefiting%20from%20them
- Stott, R. (2023, August 4). Lawsuit claims Ozempic, Mounjaro labels ‘downplayed severity’ of gastroparesis. Healio Gastroenterology. Retrieved August 11, 2023, from https://www.healio.com/news/gastroenterology/20230804/lawsuit-claims-ozempic-mounjaro-labels-downplayed-severity-of-gastroparesis#:~:text=While%20the%20drug%20label%20for,not%20recommended%20in%20these%20patients.%E2%80%9D
- How Does Gastroparesis Affect People with Diabetes? (2022, August 30). National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/professionals/diabetes-discoveries-practice/how-gastroparesis-affect-people-with-diabetes
- Living Healthy with Diabetes. (2023, March 29). www.heart.org. https://www.heart.org/en/health-topics/diabetes/prevention–treatment-of-diabetes/living-healthy-with-diabetes
- Move more; sit less. (2023, June 22). Centers for Disease Control and Prevention. https://www.cdc.gov/physicalactivity/basics/adults/index.htm#:~:text=Each%20week%20adults%20need%20150,Physical%20Activity%20Guidelines%20for%20Americans.&text=We%20know%20150%20minutes%20of,do%20it%20all%20at%20once
- Good sleep habits. (2022, September 13). Centers for Disease Control and Prevention. https://www.cdc.gov/sleep/about_sleep/sleep_hygiene.html
- Centers for Disease Control and Prevention. (2022, August 1). Food and nutrition insecurity and diabetes. Centers for Disease Control and Prevention. https://www.cdc.gov/diabetes/library/features/diabetes-and-food-insecurity.htm
Medication Assisted Treatment
- American Psychiatric Association. (2020, December). What is a substance use disorder? https://www.psychiatry.org/patients-families/addiction-substance-use-disorders/what-is-a-substance-use-disorder
- Durrani M, Bansal K. Methadone. [Updated 2023 Apr 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562216/
- Kumar R, Viswanath O, Saadabadi A. Buprenorphine. [Updated 2023 Apr 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK459126/
- National Center for Drug Abuse Statistics. (2023). Drug abuse statistics. https://drugabusestatistics.org/
- National Institute of Health. (2018, June). Understanding drug use and addiction. https://nida.nih.gov/publications/drugfacts/understanding-drug-use-addiction
- Singh D, Saadabadi A. Naltrexone. [Updated 2023 May 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK534811/
- Substance Abuse and Mental Health Services Administration. (2023, August). Methadone take-home flexibilities extension guidance. https://www.samhsa.gov/medications-substance-use-disorders/statutes-regulations-guidelines/methadone-guidance#:~:text=In%20treatment%200%2D14%20days,be%20provided%20to%20the%20patient
- S. Food and Drug Administration. (2023, May). Information about medication assisted treatment (MAT). https://www.fda.gov/drugs/information-drug-class/information-about-medication-assisted-treatment-mat
Following a DNR: An Ethical Dilemma in Nursing
- Ethics. (2021). Retrieved from https://www.dictionary.com/browse/ethics
- ANA. (2021). Retrieved from Ethics and Human Rights: https://www.nursingworld.org/practice-policy/nursing-excellence/ethics/
- Dugdale, D. C. (2020, January 12). Do-not-resuscitate order. Retrieved from MedlinePlus: https://medlineplus.gov/ency/patientinstructions/000473.htm
Managing Conflict in a Nurse Leader Role
- Johansen, M. L. (2012, February). Keeping the peace: Conflict management strategies for nurse managers. Nursing Management, 43(2).
- Kahn, M.L. et al. (2013). The modes of conflicts and managerial leadership styles of managers. Global and Business Management: An International Journal, 7(2)
- Bell, J.A. (2013). Five generations in the nursing workforce. Journal for Nurses in Professional Development 29 (4) https://www.sgna.org/Portals/0/Bell_FiveGenerationsInTheNursingWorkforce_2013.pdf
- Andrea, S. (2018, December). Embracing generational diversity: Reducing and managing workplace conflict. ORNAC Journal, ().
- Gerardi, D. (2015). Perspectives on leadership. American Journal of Nursing, 115(3), 56-61.
- McKibben, L. (2017, January). Conflict management: Importance and implications. The British Journal of Nursing, 26(2), 100 -103. doi: 10.12968/bjon.2017.26.2.100
- Kiwanuka F, Nanyonga RC, SakDankosky N, Muwanguzi PA, Kvist T. Nursing leadership styles and their impact on intensive care unit quality measures: An integrative review. Journal of Nursing Management. 2021;29:133– 142. https://doi.org/10.1111/jonm.13151
- Sherman, R. O. (2017, February). Choosing your political battles. American Nurse Today, 12(2).
- Joint Commission Resources: 2011 Hospital Accreditation Standards. Oakbrook Terrace, Ill.: Joint Commission on Accreditation of Healthcare Organizations, 2011.
- American Nurses Association. Position Statement: Incivility, Bullying, and Workplace Violence. https://www.nursingworld.org/practice-policy/nursing-excellence/official-position-statements/id/incivility-bullying-and-workplace-violence/. Published July 22, 2015. Accessed March 2, 2019.
- Gerardi, D. (2015). Conflict Engagement. American Journal of Nursing, 115(5
- Gerardi, D. (2015, August). Conflict engagement: Emotional and social intelligence. American Journal of Nursing, 115(8), 60-65.
- Gerardi, D. (2015) Conflict engagement: Connection and cultivating curiosity. American Journal of Nursing. 115(9)
- Labrague LJ, Al Hamdan Z, McEnroe– Petitte D.M. An integrative review on conflict management styles among nursing professionals: implications for nursing management. Journal of Nursing Management. 2018;26:902–917. https://pubmed.ncbi.nlm.nih.gov/30155953/
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
Click Complete
To move on to the next lesson.
