Course
Inpatient Antibiotic Therapy
Course Highlights
- In this Inpatient Antibiotic Therapy course, we will learn about three ways to classify bacteria.
- You’ll also learn the characteristics of some bacteria that might contribute to antimicrobial resistance.
- You’ll leave this course with a broader understanding of the mechanism of action of vancomycin and ceftriaxone.
About
Pharmacology Contact Hours Awarded: 2
Course By:
Charmaine Robinson
MSN-Ed, BSN, RN, PHN, CMSRN
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction
Antibiotic therapy began around 1900 after scientists noted that organisms within the soil released substances that were harmful to others (called “antibiosis”) [10]. Mimicking this process, scientists formulated the first antibiotic in the 1910s. Antibiotics have since become the gold standard for the treatment of infection. However, overuse has led to organisms fighting back.
Inappropriate prescribing of antibiotics is a major contributing factor to antibiotic resistance. For example, 70% of adults hospitalized for community-acquired pneumonia receive nearly 10 days of antibiotic therapy [2]. Yet, national guidelines recommend only five days.
More than 2.8 million antibiotic-resistant infections occur in the U.S. each year, and as a result, more than 35,000 deaths [7]. This course will take learners on a journey from the fundamentals of bacterial pathogens to the management of drug-resistant bacterial infections in inpatient settings.
Bacteria: The Basics
While there are many other pathogens that can cause infection (i.e., viruses, parasites, fungi, etc.), antibiotics treat infections caused by bacteria in particular. Finding the right treatment requires fundamental knowledge about bacteria and its characteristics.
What are Bacteria?
Bacteria are known as the earliest life forms on earth, and live in soil, seawater, people, and animals [1]. While bacteria can enter the body from an external source, many bacteria naturally live on our skin and in our airways, digestive, and genitourinary tracts – termed the “microbiome.”
Some of these bacteria are helpful to the body, for example the gut microbiome, which helps to digest food. There are as many bacteria in the microbiome as there are cells in the body [1]. Under normal conditions, these bacteria do not cause harm. However, under certain conditions, they can become pathogenic and lead to infection and disease.
Classification
Bacteria are often classified by their shape, dependency on oxygen to survive, and stains. Shapes of bacteria include cocci (spheres), bacilli (rods), and spirochetes (spirals/helixes) [1]. The ability for bacteria to survive with or without oxygen is helpful to know when selecting appropriate treatments. Aerobic bacteria need oxygen to survive. Anaerobic bacteria, on the other hand, have trouble living/growing when oxygen is present. Facultative bacteria have the ability to grow with or without oxygen [17].
In microbiology, certain chemicals (or stains) are applied to bacteria in a process called “gram staining” [1]. Bacteria are sometimes classified by the color they turn during this process. The differentiation in colors is related to the difference in the bacteria’s cell walls. Bacteria that stain blue are gram-positive. Those that stain red are gram-negative. Both types of bacteria cause different types of infections and must be treated with certain antibiotics.
Types of Bacterial Infections
The following are types of infections caused by gram negative bacteria, gram positive bacteria, spirochetes (spiral-shaped bacteria), and anaerobic bacteria [1].
Gram Negative Infections
- Escherichia coli: (found in bowels); urinary tract infections, gastroenteritis, and prostatitis (prostate infection)
- Gonorrhea: sexually transmitted infection
- Klebsiella, Enterobacter, and Serratia: (found in normal intestinal flora); affects various areas of the body
- Legionella: pneumonia
- Meningococcal: meningitis and sepsis
- Pseudomonas: affects various areas of the body
- Salmonella: foodborne; affects digestive system; can cause bacteremia
- Shigellosis: diarrhea and dysentery (a type of gastroenteritis that causes bloody diarrhea)
Gram Positive Infections
- Clostridioides difficile (c. diff): (formerly clostridium difficile); colitis
- Diphtheria: affects the upper respiratory system
- Enterococcal: (found in normal intestinal flora); urinary tract infection, bacteremia, endocarditis, cellulitis.
- Pneumococcal: pneumonia, meningitis, sinusitis, and middle ear infections
- Staphylococcal: Staph. aureus is the most dangerous; often affects the skin
- Streptococcal: affects various areas of the body
Spirochete Infections
- Syphilis: sexually transmitted infection
- Lyme disease: transmitted via a tick; affects the skin, muscles, joints, and neurological system.
Anaerobic Infections
- Botulism: foodborne; can cause paralysis
- Clostridial: (found in normal intestinal flora); enteritis and bacteremia.
- Tetanus: affects muscles (rigidity)

Self Quiz
Ask yourself...
- How often do you interpret (or review) microbiology culture reports?
- What are the most common types of bacterial infections you have encountered in your facility?
- Where do you locate antibiotic prescribing recommendations in your facility?
Antibiotic Pharmacology
Antibiotics typically work in one of two ways – by killing bacteria (bactericidal) or by inhibiting its growth (bacteriostatic) [16]. This is done through a variety of ways including:
- Inhibiting the bacteria’s cell wall development
- Increasing the bacteria cell’s membrane permeability (weakening the membrane)
- Interfering with the making of protein within the cell (which is required for cell growth)
- Affecting the metabolism of nucleic acid within the cell (which would otherwise form the DNA needed to make proteins)
- Interrupting other metabolic processes within the bacteria’s cell
This course will outline three antibiotics administered in the inpatient setting for the treatment of bacterial infections. These include piperacillin/tazobactam, ceftriaxone, and vancomycin.
Piperacillin/Tazobactam
Piperacillin/tazobactam (brand name, Zosyn) is a bactericidal combination drug that falls under the antibiotic class “beta lactam” [18]. The term beta lactam refers to the chemical structure of the drug and includes both penicillin- and cephalosporin-type antibiotics.
Piperacillin/tazobactam is a type of penicillin with a special characteristic that helps it maintain its effectiveness against drug-resistant bacteria. This medication is only administered intravenously.
Combination Therapy
Piperacillin/tazobactam is formulated as part of a two-drug combination therapy with another type of medication called a “beta lactamase inhibitor” (enzyme that enhances the effectiveness of the beta lactam antibiotic). The beta-lactamase inhibitor protects the beta lactam by preventing it from being destroyed by serine beta-lactamases (enzymes produced by some bacteria) [11][14].
With piperacillin/tazobactam, the antibiotic portion (piperacillin) fights the bacteria. The beta-lactamase inhibitor portion (tazobactam) acts as a “shield,” protecting the antibiotic from potential harm caused by the bacteria, essentially, helping to prevent antibiotic resistance.
Indication
Piperacillin/tazobactam targets both gram positive and negative aerobic and anaerobic bacteria. This medication is used to treat infections of the skin (cellulitis, abscesses, animal bites, ulcers, surgical site), abdomen (peritonitis, appendicitis, abscesses), lungs (pneumonia), and genitourinary tract (urinary tract infection, pyelonephritis, pelvic inflammatory disease). This medication is also used to treat bacteremia and sepsis and is used for surgical prophylaxis.
Mechanism of Action
As a beta lactam, piperacillin/tazobactam works by preventing bacteria from forming cell walls, which ultimately kills them. This is done by tightly binding penicillin-binding proteins within the bacteria’s cell walls, which leads to the breakdown of the cell membrane (lysis).
Pharmacokinetics
Both piperacillin and tazobactam are widely distributed into various tissues within the body, including those of the intestines, lungs, and female reproductive system. Both drugs are excreted through the kidneys.
Contraindications
Clinicians should not prescribe/administer piperacillin/tazobactam to patients with an allergy to penicillins, cephalosporins, or beta lactamases. For patients with sodium restrictions, clinicians should keep in mind that this medication contains sodium (each gram contains 65 mg [2.84 mEq] of sodium) [18].
Precaution should be taken when prescribing/administering higher doses to patients with kidney failure (may cause hematologic and nervous system impairments in this group).
Pregnancy and Breastfeeding
Penicillins (as a stand-alone drug class) are considered generally safe for use during pregnancy even though they cross the placenta. However, as a combination drug with tazobactam, studies on the effects on pregnancy are insufficient. Although penicillins enter breast milk in small amounts and can cause diarrhea and a rash in the breastfed infant, they are considered compatible with breastfeeding as long as the infant does not have a penicillin allergy. However, penicillin’s effect on the breastfed infant in combination with tazobactam is understudied.
Adverse Effects
Piperacillin/tazobactam can cause general reactions like headache, insomnia, abdominal pain, bowel elimination changes, nausea, and stomatitis (inflamed mouth and lips). With administration, clinicians should watch for hypotension, fever, and flushing. Lab changes can include high sodium, low potassium, low white blood cells, low platelets, and prolonged bleeding times. Penicillins (as with any antimicrobial drugs) can cause pseudomembranous colitis or c. diff-associated diarrhea [14].

Self Quiz
Ask yourself...
- Have you ever witnessed a patient develop an allergic reaction immediately after a parenteral dose of antibiotics?
- What questions do you ask patients about their medical history prior to prescribing/administering antibiotics?
- How comfortable are you with prescribing/administering piperacillin/tazobactam?
Ceftriaxone
Ceftriaxone (brand name, Rocephin) is a bactericidal antibiotic [15]. Like piperacillin/tazobactam, ceftriaxone falls under the antibiotic class “beta lactams” (more specifically, “cephalosporins”). Cephalosporins are characterized by generations (based on their coverage against certain bacteria). Ceftriaxone is a third-generation cephalosporin.
Indication
Similar to piperacillin/tazobactam, ceftriaxone is useful in the treatment of multiple infections, including skin infections (cellulitis, animal bites, surgical site), intrabdominal infections, pneumonia, urinary tract infection, and pyelonephritis. Ceftriaxone is also used to treat endocarditis, bacteremia, meningitis, and gonorrhea. This medication is helpful in the treatment of gram-negative organisms.
Mechanism of Action
As mentioned earlier, beta lactam antibiotics work by binding to penicillin-binding proteins within the bacteria’s cell walls, leading to breakdown of the cell membrane, and ultimately death of the cell.
Pharmacokinetics
Ceftriaxone is widely distributed into most tissues, including gallbladder, liver, kidney, bone, uterus, and ovary [15]. This medication is also distributed into biliary, peritoneal, pleural, and synovial (joint) fluids. Ceftriaxone is unique as it can penetrate inflamed meninges, reaching therapeutic levels within the cerebral spinal fluid (making it a great treatment option for meningitis). Ceftriaxone is excreted via urine and stool.
Contraindications
Ceftriaxone should not be prescribed/administered to patients with an allergy to cephalosporins or cephamycin. Additionally, this medication should not be prescribed/administered with calcium-containing intravenous solutions or products. Precautions should be taken in patients with an allergy to penicillin as the structure of both medications are similar and cross-sensitivity may occur.
Pregnancy and Breastfeeding
Although ceftriaxone crosses the placenta, it does not show drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes during pregnancy. In general, cephalosporins are considered safe for use during pregnancy. There are no studies on ceftriaxone’s effect on breastfed infants. However, this medication can enter breast milk and potentially alter the infant’s developing gut flora (rare).
Adverse Reactions
Ceftriaxone can cause abdominal symptoms (c. diff colitis, upset stomach, stomatitis), lab changes (elevated liver enzymes, anemia, low white blood cells, low platelets), neurological symptoms (lethargy, seizures), and allergic reactions (itching, rash).

Self Quiz
Ask yourself...
- How often do you prescribe/administer parenteral antibiotics in your facility?
- How frequently do you monitor basic labs when a patient is prescribed antibiotics? What lab values do you monitor?
- How comfortable are you with prescribing/administering antibiotics to pregnant patients?
- Do you collaborate with infectious disease specialists when prescribing antibiotics (or have you witnessed a provider doing so)?
Vancomycin
Vancomycin is a strong bactericidal antibiotic [19]. While most commonly administered in its parenteral form, vancomycin may be administered in an oral suspension liquid. Vancomycin falls under the antibiotic class “glycopeptides.”
Indication
Vancomycin is useful in the treatment of infections caused by gram-positive cocci and bacilli only, including staph. aureus and coagulase-negative staph strains resistant to beta-lactams. Vancomycin also targets many strains of enterococci, and other drug-resistant bacteria. This medication is commonly used to treat serious infections and endocarditis. The oral form of vancomycin is used to treat c. diff colitis.
Mechanism of Action
Vancomycin works by preventing bacteria from forming cell walls, which ultimately kills them. More specifically, the drug works on a structure within the bacteria’s cell wall called “peptidoglycan” (hence the drug class name “glycopeptide”). Peptidoglycan is a large molecule consisting of sugars and amino acids that forms a mesh-like layer that lines the cell’s membrane. Vancomycin binds to a bacterial enzyme (D-alanyl D-alanine) within the cell wall, inhibiting the transfer of the amino acids necessary to develop the peptidoglycan. This leads to weakening of the bacteria’s cell wall (increased permeability), which causes cell contents to leak outside of the cell (ultimately killing the cell) [13].
Pharmacokinetics
Oral vancomycin is not absorbed well in a normal gastrointestinal (GI) tract and therefore is not ideal for treating systemic infections. Parenteral forms penetrate well into bile, and pleural, pericardial, synovial, and ascitic (abdominal) fluids. In patients with normal kidney function, parenteral vancomycin has a serum half-life of 4 to 6 hours. Parenteral vancomycin is primarily excreted in urine (within 24 hours). Oral vancomycin is primarily excreted in stool.
Contraindications
Vancomycin should not be prescribed/administered to patients with an allergy to the drug. Clinicians should consider dose reductions in patients with kidney disease as nephrotoxicity can occur.
Pregnancy and Breastfeeding
Studies are minimal on the effects of vancomycin on pregnancy. However, experts recommend administering the medication to pregnant patients only if absolutely necessary. Oral vancomycin may be prescribed/administered to patients who are pregnant.
Vancomycin is discouraged in patients who are breastfeeding as the drug enters breast milk and can disrupt the infant’s developing gut flora. As mentioned earlier, oral vancomycin absorption is poor in a normal GI tract, therefore systemic adverse effects in infants are not likely.
Adverse Reactions
The most common adverse effect caused by vancomycin is an allergic reaction. For this reason, vancomycin should be infused slowly in a diluted solution over 60 minutes or longer [19]. Vancomycin infusion can cause a histamine-mediated reaction (itching and flushing on the face/neck/shoulders). Rash or fever can also occur, particularly when the drug course is greater than two weeks. Clinicians might expect to see lab changes (low platelets and low white blood cells). Although rare, nephrotoxicity can occur with high doses and when given with other nephrotoxins. Some studies even suggest vancomycin use in combination with piperacillin/tazobactam may increase the risk of nephrotoxicity.

Self Quiz
Ask yourself...
- Why do you think it is important to know vancomycin’s mechanism of action? How might this information influence prescribing/administration?
- How often have you administered oral vancomycin for c. diff?
- What is the most common indication for which you have prescribed vancomycin (or witnessed a provider doing so)?
- Have you ever encountered a patient who developed nephrotoxicity from parenteral antibiotic use?
Antimicrobial Resistance
Antimicrobial/antibiotic resistance has been on the rise [10]. After discovery of the first antibiotic in the early 1900s, antimicrobial resistance was shortly to follow as just 10 years later, resistance was identified. Around that time, sulfonamides – another group of synthetic antibiotics – were discovered, along with penicillin. Less than 10 years later, resistance was identified to both.
In the fight for survival, organisms are finding ways to outsmart the mechanisms of antibiotic therapy.
What is Happening at the Cellular Level?
Bacteria have several defenses that help them to survive [1]. Biofilm is a sticky layer on some bacteria formed by substances released by the bacteria that helps them attach to other cells. Biofilm makes it difficult for antibiotics to kill the bacteria. Another protective mechanism of some bacteria is encapsulation. The bacteria are protected inside of a capsule that prevents immune cells from destroying them. Similar to encapsulation, some bacteria (gram-negative) are protected by an outer membrane. When disrupted, the membrane releases endotoxins (toxic substances) that worsen the infection. Other bacteria release enzymes that destroy the antibiotics meant to kill them.
Spore formation is another way bacteria survive. Some bacteria produce spores (inactive forms of the bacteria) which help them to survive in difficult conditions. Once conditions are better, the spores become active. Other bacteria become resistant by simply acquiring genes from bacteria that are already resistant. These genes can be passed on through generations or transferred interspecies.
Statistical Evidence
In 2019, an estimated 4.95 million deaths worldwide were associated with bacterial antimicrobial resistance [12]. Lower respiratory infections were among the most common, accounting for 1.5 million deaths. The U.S. tracks many drug-resistant bacterial infections. However, not all bacterial infections are considered invasive (serious). The U.S. closely monitors several bacteria that cause invasive bacterial infections – the top three includes Group B Streptococcus, Group A Streptococcus, and Streptococcus pneumoniae [See Figure 1] [9].

Figure 1. CDC Active Bacterial Core Surveillance Report [9]
Historical Timeline of Antibiotic Resistance
One of the most notable antibiotic-resistant organisms is Staphylococcus aureus, more specifically Methicillin-resistant Staphylococcus aureus (MRSA). MRSA is very difficult to treat and typically causes skin infections. However, in healthcare settings MRSA can also cause pneumonia and surgical site infections [4]. If left untreated, MRSA can lead to sepsis. MRSA was first identified in the 1960s.
Vancomycin, often used to treat MRSA, was introduced in the 1970s and due to its overuse, organisms began to develop resistance rather quickly. Vancomycin resistance was identified in the late 1980s from a group of organisms called enterococcus, giving way to VRE (Vancomycin-resistant Enterococcus). Enterococcus is typically found in the bowels and in the female genitourinary tract, and related infections often occur in healthcare settings [5]. In the early 2000s, staph eventually developed a resistance to Vancomycin as well (VRSA).
Due to the highly resistive nature of some bacteria, treatment began to expand to several classes of antibiotics over the past 10 years, but as nature would have it, the presence of multi-drug-resistance organisms (MDROs) began to emerge [10]. As scientists continue to develop new antibiotics to treat illnesses, organisms continue to fight back.
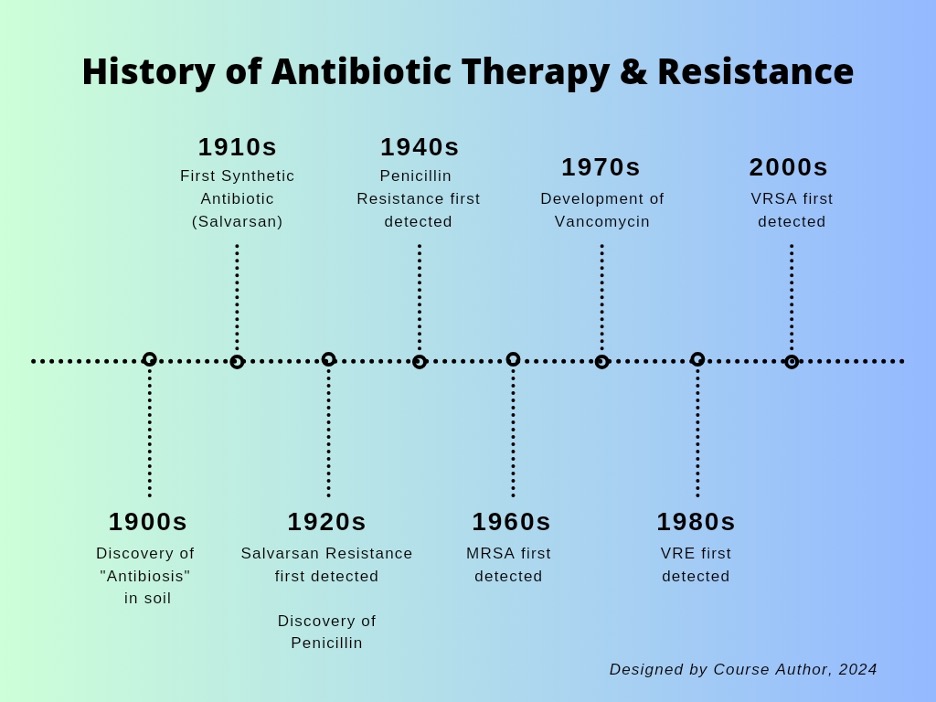
Figure 2. Historical Timeline of Antibiotic Therapy and Resistance [10]

Self Quiz
Ask yourself...
- How often have you witnessed a patient experience worsening symptoms of an infection even after antibiotic therapy?
- What do you think are the determining factors for why an infection may progress to an invasive one?
- The most common age for acquiring a serious bacterial infection is between 50 to 64. What do you think are contributing factors?
- What do you think was the primary mechanism of antimicrobial resistance in historical times?
Common Drug-Resistant Bacteria
The CDC outlines 18 antimicrobial-resistant bacteria under national surveillance as of 2019. The bacteria are categorized according to the level of threat.
These include urgent, serious, concerning, and watch list [6].
Urgent Threats
|
U.S. Antimicrobial Resistant Bacteria: Urgent Threats |
||
|
Bacteria |
Cases |
Deaths |
|
Carbapenem-resistant Acinetobacter |
8,500 (in 2017) |
700 (in 2017) |
|
Clostridioides difficile |
223,900 (per year) |
12,800 (per year) |
|
Carbapenem-resistant Enterobacterales |
13,100 (in 2017) |
1,100 (in 2017) |
|
Drug-resistant Neisseria gonorrhoeae |
550,000 (per year) |
|
Serious Threats
|
U.S. Antimicrobial Resistant Bacteria: Serious Threats |
||
|
Bacteria |
Cases |
Deaths |
|
Drug-resistant Campylobacter |
448,400 (per year) |
70 (per year) |
|
Extended-spectrum beta-lactamase (ESBL)-producing Enterobacterales |
197,400 cases (in 2017) |
9,100 (in 2017) |
|
Vancomycin-resistant Enterococci (VRE) |
54,500 (in 2017) |
5,400 (in 2017) |
|
Multidrug-resistant Pseudomonas aeruginosa |
32,600 (in 2017) |
2,700 (in 2017) |
|
Drug-resistant nontyphoidal Salmonella |
212,500 (per year) |
70 (per year) |
|
Drug-resistant Salmonella serotype Typhi |
4,100 (per year) |
Less than 5 (per year) |
|
Drug-resistant Shigella |
77,000 (per year) |
Less than 5 (per year) |
|
Methicillin-resistant Staphylococcus aureus (MRSA) |
323,700 (in 2017) |
10,600 (in 2017) |
|
Drug-resistant Streptococcus pneumoniae |
900,000 (in 2014) |
3,600 (in 2014) |
|
Drug-resistant Tuberculosis |
847 (in 2017) |
62 (in 2017) |
Concerning Threats
|
U.S. Antimicrobial Resistant Bacteria: Concerning Threats |
||
|
Bacteria |
Cases |
Deaths |
|
Erythromycin-Resistant Group A Streptococcus |
5,400 (in 2017) |
450 (in 2017) |
|
Clindamycin-resistant Group B Streptococcus |
13,000 (in 2016) |
720 (in 2016) |
Watch List
|
U.S. Antimicrobial Resistant Bacteria: Watch List |
||
|
Bacteria |
|
|
|
Drug-resistant Mycoplasma genitalium (sexually transmitted infection) |
||
|
Drug-resistant Bordetella pertussis (“whooping cough”) |
||

Self Quiz
Ask yourself...
- Why do you think c. diff has the highest mortality rate of any multidrug resistant bacteria in the U.S.?
- Aside from c. diff and MRSA, what is another type of drug-resistant bacteria you have frequently encountered in your facility?
- In what care setting might you anticipate a high number of patients with “whooping cough”?
- Which drug-resistant bacteria do you think will cause a growing number of infections over the next five years? Why?
Guidelines for Management of Drug-Resistant Pathogens
Guidelines are practices that are set forth by governing bodies, both national and global, to ensure patient safety and high-quality care. Antibiotic guidelines assist facilities and organizations in better prescribing practices and patient use of antibiotics.
The CDC originally introduced “Infection Control Guidelines” to help healthcare organizations prevent the spread of organisms. However, due to the surge of difficult-to-treat infections caused by highly threatening antibiotic-resistant organisms, additional guidelines have been introduced to try to “tame the beast” so-to-speak. In 2006, the CDC introduced antibiotic resistance guidelines, comprised of the “Multi-Drug Resistant Organism (MDRO) Management Guidelines” and the “Containment Strategy Guidelines.”
This course will highlight the “Containment Strategy Guidelines.”
Containment Strategy Guidelines
The “Containment Strategy Guidelines,” formally the “Interim Guidance for a Public Health Response to Contain Novel or Targeted Multidrug-resistant Organisms (MDROs),” is a set of general guidelines to assist providers and clinicians to [3]:
- Identify affected patients.
- Ensure control measures are implemented.
- Determine if transmission is occurring between facilities.
- Identify new organisms and mechanisms in order to guide treatment.
- Engage in prevention activities.
Inpatient health care facilities can use these practice guidelines when encountering a patient with MDRO. The guidelines are unique to specified “tiers” (or levels of epidemic stages). These include:
- Tier 1 (Limited Spread): organisms rarely identified in the U.S.
- Tier 2 (Limited-to-Moderate Spread): organisms not regularly found in a particular region, but have the potential to spread
- Tier 3 (Moderate-to-Advanced Spread): organisms regularly found in a region, but not endemic
- Tier 4 (Endemic): organisms prevalent in a particular region
For the sake of this course, guidelines will be outlined for Tier 3 and will specify practices that apply to nurse clinicians/leaders.
Upon receiving laboratory results of MDRO, the following steps should be applied:
- Initiate Response Measures
- Promptly notify the primary care provider, patient care personnel, infection control departments, and other healthcare staff per facility policy.
- Notify state and local health departments (depending on local regulations)
- Implement appropriate infection control measures (i.e., contact precautions)
- Notify the patient and family about the results and infection control measures being implemented.
- If the MRDO is present on admission, the healthcare facility notifies the transferring facility so infection control measures can be taken at that facility as well.
- Conduct a Healthcare Investigation
- Review the patient’s healthcare exposures (e.g., office visits, home health visits) prior to the testing results up until the present, including overnight stays in healthcare settings.
- Conduct a Contact Investigation
- Screen patients according to health department recommendations (these should consider local epidemiology, laboratory capacity, and ongoing prevention activities and objectives).
- Consider screening individuals who have extensive household contact with the patient (shares a bed or assists with cares) if they have frequent healthcare exposures, solely for the purpose of determining the individual should be placed on transmission-based precautions in subsequent admissions.
- Implement a System to Ensure Adherence to Infection Control Measures
- Educate healthcare personnel and patient visitors about the organism and precautions taken.
- Ensure that adequate supplies are available to implement precautions.
- Conduct ongoing evaluations of infection control practices and provide feedback to healthcare personnel.
- Flag medical records of affected patients to ensure implementation of appropriate infection control precautions upon the patient’s readmission.
- Plan for how to inform receiving facilities of patient’s MDRO status when transferred to a new facility.
- Do not discharge a patient from one level of care to another (to a new department or facility) based on colonization (whether a patient is infected or not).

Self Quiz
Ask yourself...
- Within what time frame must the lab notify the care team of new drug-resistant bacteria results in your facility?
- In your facility, how long do patients remain on contact precautions for an active MRSA infection?
- In your facility, what is the protocol for notifying receiving facilities of a patient’s active MRSA status (when transferring the patient to a new facility)?
- Do you repeat microbiology cultures during/after prescribing a course of antibiotics (or have you witnessed a provider doing so)?
Guidelines for Hospital Antibiotic Stewardship
The CDC defines antibiotic stewardship as the “effort to measure and improve how antibiotics are prescribed by clinicians and used by patients,” [8, para. 1]. Antibiotic stewardship guidelines are primarily geared towards leaders within a facility or organization (i.e., medical directors, lead pharmacists, nursing leaders).
While these guidelines may not be directed towards nurse clinicians working in patient-facing roles, it is important to understand what is happening at the upper management level as this can prepare them for practice changes that may take place in the workplace.
The CDC’s “Core Elements of Antibiotic Stewardship” is a guideline that outlines recommendations for facilities and organizations to improve antibiotic prescribing and use in certain care settings, including health departments, hospitals, outpatient care, and nursing homes. The ultimate goal is to effectively treat infections within the facility/organization, ensure antibiotic safety for patients, and tackle antimicrobial resistance. This course will focus on the guidelines for hospitals.
The “Core Elements of Hospital Antibiotic Stewardship” include seven main focal points [8]:
- Hospital Leadership Commitment
Hospital leadership should be committed to antibiotic stewardship by facilitating the resources needed to maintain antibiotic stewardship (e.g., adequate staffing, financial support). Leaders can include clinicians, department heads, pharmacists, infection preventionists and epidemiologists, quality improvement teams, microbiology staff, information technology staff, and nurses.
- Accountability
Hospitals should appoint a leader to guide the antibiotic stewardship program. Clear responsibilities and expectations of the team should be outlined, and team rounding should be implemented to discuss progress.
- Pharmacy Expertise
Hospitals should employ pharmacists specialized in infectious disease. The pharmacist should be either the lead or co-lead for the team.
- Action
Hospitals should take action by first assessing antibiotic prescribing within the facility and implementing auditing and feedback by an external expert in antibiotic use (to ensure prescribed treatments are in line with appropriate guidelines). Hospitals should also consider implementing “preauthorization” (a process in which clinicians must receive approval prior to prescribing a particular antibiotic). Lastly, hospitals might consider implementing antibiotic “timeouts” (a process in which clinicians reassess the continued need for antibiotics after repeat labs/cultures).
- Tracking
Hospitals should track antibiotic use measures, outcome measures (e.g., monitoring infection rates, pathogen resistance to antibiotics, and financial costs, etc.), and process measures (e.g., monitoring how well staff adheres to all actions implemented above, etc.).
- Reporting
Hospitals should share facility-specific information on antibiotic use/resistance to providers, pharmacists, nurses, and leadership within the facility.
- Education
Hospitals should educate staff on antibiotic use and optimal prescribing in formal and informal settings, and through presentations (case presentations might be most beneficial), flyers/posters, and electronic communication to staff (like newsletters). Hospitals should also ensure that patients are educated on the antibiotics they are prescribed, including indications and side effects.


Self Quiz
Ask yourself...
- How do leaders in your facility track antibiotic prescribing/administration practices?
- When prescribing/administering antibiotics in your facility, what types of associated documentation must be included in the medical record?
- How often does your facility offer training on antibiotic prescribing/administration or how to manage drug-resistant bacteria?
- Do you have any ideas on how to improve antibiotic prescribing/administration in your facility?
Conclusion
Prior to the discovery of antibiotics in the early 1900s, serious infections most likely meant a death sentence. Although antibiotics have extended the life expectancy of humans by 23 years, antimicrobial resistance has led to many deaths [10].
Nurse clinicians working in inpatient settings can contribute by staying up to date on antibiotic pharmacology, studying drug-resistant pathogens, following clinical guidelines on safe prescribing/administration practices, and preventing the spread of these difficult-to-treat organisms.
References + Disclaimer
- Bush, L. M. (2022, September). Overview of Bacteria. https://www.msdmanuals.com/home/infections/bacterial-infections-overview/overview-of-bacteria
- Centers for Disease Control and Prevention. (2018). Antibiotic use in the United States, 2018 update: Progress and opportunities. https://www.cdc.gov/antibiotic-use/stewardship-report/pdf/stewardship-report-2018-508.pdf
- Centers for Disease Control and Prevention. (2023, March). Containment strategy: Interim guidance for a public health response to contain novel or targeted multidrug-resistant organisms (MDROs). https://www.cdc.gov/hai/mdro-guides/containment-strategy.html
- Centers for Disease Control and Prevention. (2019, June). Methicillin-resistant Staphylococcus aureus (MRSA): General Information. https://www.cdc.gov/mrsa/community/index.html
- Centers for Disease Control and Prevention. (2019, November). Vancomycin-resistant enterococcus (VRE) in healthcare settings. https://www.cdc.gov/hai/organisms/vre/vre.html
- Centers for Disease Control and Prevention. (2021, November). 2019 AR threats report. https://www.cdc.gov/drugresistance/biggest-threats.html
- Centers for Disease Control and Prevention. (2021, November). Be antibiotic aware: Smart use, best care. https://www.cdc.gov/patientsafety/features/be-antibiotics-aware.html
- Centers for Disease Control and Prevention. (2023, September). Core elements of antibiotic stewardship. https://www.cdc.gov/antibiotic-use/core-elements/index.html
- Centers for Disease Control and Prevention. (2023, September). Surveillance reports. https://www.cdc.gov/abcs/reports-findings/surv-reports.html
- Hutchings, M. I., Truman, A. W., & Wilkinson, B. (2019). Antibiotics: past, present and future. Current Opinion in Microbiology, 51, 72–80. https://doi.org/10.1016/j.mib.2019.10.008
- Khanna, N.R., & Gerriets, V. (2022). Beta-lactamase inhibitors. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK557592/
- Murray, C. J. L., Ikuta, K. S., Sharara, F., et al. (2022). Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. The Lancet, 399(10325), P629-655. https://doi.org/10.1016/S0140-6736(21)02724-0
- Patel, S., Preuss, C.V., & Bernice, F. (2023, March). Vancomycin. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK459263/
- Robinson, C. (2024). Guidelines for outpatient antibiotics. https://nursingcecentral.com/view-courses/
- Werth, B. J. (2022, September). Cephalosporins. Merck Manual Professional Version. https://www.merckmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-drugs/cephalosporins
- Werth, B. J. (2022, September). Overview of Antibacterial Drugs. Merck Manual Professional Version. https://www.merckmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-drugs/overview-of-antibacterial-drugs
- Werth, B. J. (2022, September). Overview of Bacteria. Merck Manual Professional Version. https://www.merckmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-drugs/overview-of-bacteria
- Werth, B. J. (2022, September). Penicillins. Merck Manual Professional Version. https://www.merckmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-drugs/penicillins
- Werth, B. J. (2022, September). Vancomycin. Merck Manual Professional Version. https://www.merckmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-drugs/vancomycin
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click the Green MARK COMPLETE Button Below
To receive your certificate
