Course
Malignant Brain Tumor Review
Course Highlights
- In this Malignant Brain Tumor Review course, we will learn about the cellular mechanisms underlying malignant brain tumors.
- You’ll also learn contributing factors including tumor location, growth rate, and surrounding brain structures influence the presentation of symptoms.
- You’ll leave this course with a broader understanding of various treatment modalities, including surgery, radiation, chemotherapy, and emerging therapies like cell-based and gene therapies.
About
Contact Hours Awarded: 2
Course By:
R.E. Hengsterman MSN, RN
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction
Malignant brain tumors are aggressive cancers that exhibit rapid growth and can spread to other parts of the brain and spinal cord [1]. Cancerous tumors, which form when genes regulating cell growth malfunction, can develop anywhere in the body, including the brain and spinal cord, and may displace or disrupt healthy cells depending on their origin type [1].
The World Health Organization (WHO) developed a comprehensive grading tumor grading system, ranging from 1 to 4, to help assess tumor aggressiveness and determine the most appropriate treatment strategies [13]. In some cases, malignant brain tumors may be a combination of different tumor types or may originate from various areas within the brain. Malignant tumors, classified as grade 3 or 4, exhibit rapid growth rates and a high likelihood of recurrence after treatment. In contrast, benign (non-cancerous) tumors, often graded as 1 or 2, exhibit slow growth and carry a lower risk of recurrence [2] [3].
A brain tumor, also known as an intracranial tumor, is an abnormal mass of tissue resulting from uncontrolled cell multiplication [4]. There are more than 150 distinct types of brain tumors, categorized into primary and metastatic tumors [4]. Primary brain tumors start within the brain itself, while most malignant brain tumors are secondary cancers that originate in another part of the body and metastasize to the brain [5].
The prevalence of brain tumors underscores the need for general medical providers to have a fundamental understanding of their diagnosis and management. Common types include intracranial metastases from systemic cancers, meningiomas, gliomas, and glioblastomas—the latter being the most common and aggressive malignant primary brain tumor [5].
Symptoms of malignant brain tumors vary depending on their size and location but often include severe, persistent headaches; seizures; persistent nausea, vomiting, and drowsiness; mental or behavioral changes like memory problems or personality shifts; progressive weakness or paralysis on one side of the body; and vision or speech difficulties [6].
Advancements in treatment have led to new frontiers involving cell-based and targeted therapies. Active and adoptive immunotherapies, stem cell therapies, and gene therapies have evolved due to extensive preclinical and clinical studies. Clinical trials have validated the effectiveness of antibody-based immunotherapies—such as bevacizumab—when combined with standard care [7]. Preclinical data also highlight the potential of vaccines, engineered cells, and gene therapies in preventing tumor recurrence [8].
A comprehensive understanding of the most common brain tumors—including their diagnosis, oncologic management, and handling of medical complications—is crucial. The new Cryosection Histopathology Assessment and Review Machine (CHARM) classification of gliomas incorporates both molecular features and histology to provide an integrated diagnosis, offering better prognostic insights [9]. Understanding these aspects is essential for improving patient outcomes and developing tailored treatment approaches for both new and recurrent gliomas.

Self Quiz
Ask yourself...
- How might the location and size of a brain tumor influence the specific symptoms a patient experiences, and why would these symptoms vary so widely?
- In what ways could cell-based therapies and gene therapies alter the standard approach to treating brain tumors, reducing recurrence, and improving outcomes?
- How does the CHARM classification system improve the understanding and treatment of gliomas, and what potential advantages does this integrated approach offer over previous classification methods?
Primary Brain Tumors
Primary brain tumors originate within the brain or its immediate surroundings. They include glial and non-glial types and may be benign or malignant [4]. Glial tumors arise from the brain’s supportive tissues, while non-glial tumors develop within other structures of the brain, such as nerves, blood vessels, or glands [10]. Glial tumors originate in the brain’s supportive tissues, which play a critical role in nourishing, insulating, and protecting neurons. Their presence can significantly disrupt these essential support functions [10].
As glial tumors grow, they can interfere with nutrient supply, waste removal, and signal insulation, leading to a range of neurological deficits. This disruption can affect motor control, cognitive functions, sensory processing, and even alter personality, depending on the tumor’s location and the extent to which it compromises these supportive tissues [10].

Self Quiz
Ask yourself...
- What differences might exist in the behavior and treatment of glial versus non-glial brain tumors, and how could their origins in distinct brain structures impact patient outcomes?
- How might the disruption of supportive functions by glial tumors impact a person’s neurological abilities, and why would the specific location of the tumor lead to varied symptoms across different individuals?
Metastatic Brain Tumors
Metastatic brain tumors originate in other parts of the body, such as the breast or lungs, and spread to the brain often through the bloodstream [11]. Metastatic brain tumors affect one in four cancer patients, with around 150,000 new cases per year [11] [12]. For example, up to 40% of individuals with lung cancer will develop metastatic brain tumors [4].
While the prognosis for these tumors has been poor, recent advances in diagnostic techniques, surgical interventions, and radiation therapies have improved survival rates and quality of life.

Self Quiz
Ask yourself...
- How might the origin of metastatic brain tumors in other parts of the body, like the lungs or breast, influence the treatment approach and prognosis compared to primary brain tumors?
Tumor Staging
The World Health Organization (WHO) classifies tumors based on their microscopic histological features, indicating whether they are malignant or benign [13]. Key characteristics of high-grade, malignant tumors include:
- A high degree of malignancy
- Rapid growth and aggressive behavior
- Extensive infiltration into surrounding tissues
- High likelihood of recurrence
- Prone to necrosis (tissue death) Top of Form
Types of Malignant Tumors
Most malignant brain tumors originate from the glial tissue, which provides support to the brain’s nerve cells [4]. These tumors, gliomas, have types based on the cells they arise from. Examples include. Brain tumors include gliomas, which account for 78% of all cases [4].
Gliomas develop from glial cells, which are the supportive cells of the brain, including astrocytes, oligodendrocytes, and ependymal cells [14].

Self Quiz
Ask yourself...
- How does the WHO grading system aid in determining the prognosis and treatment plan for a patient with a brain tumor, and what implications does the tumor’s grade have on its behavior and recurrence?
- Given that most malignant brain tumors originate from glial cells, how might the specific type of glial cell involved (e.g., astrocytes, oligodendrocytes, ependymal cells) affect the tumor’s growth pattern and impact on brain function?
Symptomology
Symptoms of brain tumors vary depending on their location, size, and rate of growth. Common signs include persistent headaches that may worsen over time, often more intense in the morning or severe enough to awaken the individual at night [6] [15]. Blurred or double vision can occur due to increased intracranial pressure.
Patients experience nausea, vomiting, and loss of appetite, leading to weight loss and dehydration [6][15]. Cognitive impairments such as memory loss, difficulty concentrating, confusion, and problems with thinking, speaking, or articulating thoughts may arise when tumors impact cognitive processing regions [6][15]. Mood or personality changes are also common, as tumors affecting areas responsible for emotion and behavior can result in irritability, depression, or altered social interactions [6] [15][16].
The sudden onset of seizures or convulsions is a significant symptom, caused by abnormal brain tissue disrupting normal electrical activity [17].
Additional symptoms may include weakness or paralysis affecting one side of the body, loss of balance or dizziness, hearing changes, facial numbness or tingling, swallowing difficulties, and speech difficulties like slurred speech or trouble finding the right words [6] [15][16]. The presence of these symptoms warrants prompt medical evaluation, as early detection and treatment of brain tumors can improve outcomes and prevent further complications.

Self Quiz
Ask yourself...
- How might the specific location and growth rate of a brain tumor contribute to differences in symptoms like cognitive impairment, mood changes, or motor deficits among patients?
- In what ways could early detection of symptoms, such as persistent headaches or sudden seizures, influence the treatment approach and overall prognosis for individuals with brain tumors?
Astrocytoma
Astrocytomas are a significant subset of brain tumors originating from astrocytes—the star-shaped glial cells that provide structural and metabolic support to neurons in the central nervous system (CNS) [18]. Astrocytomas affect the brain but can also involve the cord [18]. Glial tumors constitute about 60% of brain tumors, with astrocytomas among them [18]. These tumors pose a considerable clinical challenge due to their prevalence and the serious morbidity and mortality they cause across all age groups. Early diagnosis and prompt treatment are crucial for improving patient outcomes.
Astrocytomas originate from astrocytes, star-shaped cells that provide structural support to the brain [18][19]. They can develop in regions of the brain but develop most often in the cerebrum. While they are more frequent in middle-aged men, astrocytomas can also occur in children, where they are low-grade [18][19]. In adults, astrocytomas tend to be more aggressive [14][18].
The exact cause of astrocytoma remains unknown, with exposure to ionizing radiation being the only well-established risk factor [18]. Children who receive prophylactic radiation therapy for conditions like acute lymphocytic leukemia have an increased risk—up to 22 times—of developing CNS malignancies within 5 to 10 years after treatment [20] [21]. In addition, radiation therapy for pituitary adenomas has been associated with a 16-fold increased risk of glioma development [22].
Researchers have studied associations with risk factors such as fields (e.g., from phones), head injuries, or exposures, but we have no evidence supporting these links [23]. A minority of cases show a familial predisposition in genetic conditions like Turcot syndrome, Li-Fraumeni syndrome (p53 mutations), and neurofibromatosis type 1 (NF1). Notably, about 66% of low-grade astrocytomas demonstrate p53 mutations [24] [25].
Understanding the epidemiological patterns of astrocytoma is important for identifying risk factors and enhancing early detection efforts across different populations. Researchers observe minimal racial differences in astrocytoma incidence [18]. In terms of gender, pilocytic astrocytomas show no significant dominance, but studies indicate a slight male predominance in low-grade astrocytomas, with a male-to-female ratio of 1.18:1 [18] [26]. Anaplastic astrocytomas show a more pronounced male predominance, with a male-to-female ratio of about 1.87:1 [18] [26]. Age-wise, pilocytic astrocytomas are more common in the first two decades of life [18] [26]. Astrocytomas affect individuals between 30 and 40 years old, accounting for about one-fourth of cases [26]. For low-grade astrocytomas, 10% of patients are younger than 20 years, 60% are between 20 and 45 years, and 30% are older than 45 years [27]. For grade III astrocytomas (anaplastic astrocytomas), the average age at diagnosis is around 40 years [26][27].
The tumor’s local effects are due to multiple mechanisms, including direct invasion of surrounding brain tissue, leading to disruption of normal function, and competition with normal tissue for oxygen and nutrients, resulting in hypoxic injury [28]. The tumor microenvironment—comprising interactions with other cell types, extracellular matrix components, and blood vessels—also contributes to tumor progression. Molecular alterations such as genetic mutations and changes in signaling pathways, particularly the PI3K/AKT/mTOR and MAPK pathways, promote uncontrolled cell growth and survival [28] [29]. These mutations can disrupt normal cellular processes like cell cycle regulation, apoptosis, and differentiation.
The complexity of these mechanisms contributes to the heterogeneity and therapeutic resistance often seen in astrocytoma cases [18][19]. Astrocytomas vary in aggressiveness, ranging from slow-growing pilocytic astrocytomas to malignant glioblastomas [18] [28]. The mass effect of the tumor can lead to various clinical signs and symptoms due to increased intracranial pressure and localized brain dysfunction. Understanding the molecular and cellular mechanisms underlying astrocytoma development is essential for advancing diagnostic methods and developing targeted therapies.

Self Quiz
Ask yourself...
- How might the origin of astrocytomas in astrocytes, which provide structural and metabolic support to neurons, influence the symptoms and challenges associated with these tumors?
- In what ways could factors such as age, gender, or prior radiation exposure impact the development and progression of astrocytomas, and why might certain populations be more at risk?
- How do molecular and genetic mutations within astrocytomas, such as changes in signaling pathways like PI3K/AKT/mTOR, contribute to the tumor’s aggressiveness and resistance to treatment?
Oligodendroglioma
Originates from cells responsible for producing the fatty myelin sheath covering nerves [5]. Oligodendroglioma is a primary central nervous system (CNS) tumor that originates in the brain or spinal cord [30]. Diagnosis requires the identification of two specific genetic alterations: a mutation in the isocitrate dehydrogenase (IDH) gene and a co-deletion of the short arm of chromosome 1 and the long arm of chromosome 19, known as a 1p/19q codeletion [31]. Accurate diagnosis involves the surgical removal of tumor tissue for analysis by a neuropathologist.
Pathologists classify these tumors into two grades based on their characteristics:
- Grade II Oligodendrogliomas: These are low-grade tumors that are slow growing and invade nearby normal tissue [30]. They often develop over several years before symptoms become apparent, leading to a delayed diagnosis.
- Grade III Oligodendrogliomas (Anaplastic Oligodendrogliomas): These are tumors that exhibit rapid growth [30].
On magnetic resonance imaging (MRI), oligodendrogliomas appear as a single tumor with well-defined borders, often show contrast enhancement, and may exhibit surrounding swelling [30]. Researchers do not know the exact cause of oligodendrogliomas. Cancer arises due to genetic mutations that alter cell function, increasing the growth and spread of cancer cells [32]. Oligodendrogliomas form in the white matter and the cortex, the outer layer of the brain, but they can develop anywhere within the CNS. Scientists name them for their resemblance to oligodendrocytes, cells that support and insulate fibers. Although these tumors can spread to other areas of the CNS through cerebrospinal fluid (CSF), such occurrences are uncommon, and they do not often metastasize to other organs [30][33].
Symptoms depend on the tumor’s location. The most common sign is a seizure, experienced by about 60% of patients before diagnosis [30]. Other symptoms may include headaches, cognitive difficulties, weakness, numbness, and problems with balance and movement [30].
Oligodendrogliomas develop in people aged 35 to 44 but occur at other ages [30]. They are more prevalent in males and are rare in children [30]. In the United States, an estimated 14,950 people live with this tumor, and a population-based analysis of 244,808 glioma patients found that non-Hispanic white individuals have the highest incidence and lowest relative survival rates across most histology [34].

Self Quiz
Ask yourself...
- How might genetic mutations, such as IDH mutation and 1p/19q codeletion, impact both the diagnosis and treatment of oligodendrogliomas compared to other CNS tumors?
- In what ways could the slow-growing nature of Grade II oligodendrogliomas influence the timing of symptom onset and subsequent treatment decisions?
- How does the location of oligodendrogliomas in the white matter and cortex contribute to common symptoms like seizures and cognitive difficulties, and why might these symptoms vary between individuals?
Ependymoma
Ependymoma is a primary central nervous system (CNS) tumor originating in the brain or spinal cord [35][36]. Diagnosis involves the surgical removal of tumor tissue for analysis by a neuropathologist to ensure accuracy. Doctors classify ependymomas into three grades based on characteristics and behavior. Grade I ependymomas, which are low-grade tumors, and are less aggressive. Subependymomas, a subtype of grade I ependymomas, can arise in the brain or spine and are more common in adults than in children [35][36].
Grade II ependymomas are also low-grade and slow growing but have a greater recurrence than grade I tumors, if not removed [35][36]. Subtypes of grade II ependymomas include myxopapillary ependymoma, which arises in the spine and is more common in adults, and conventional grade II ependymoma, which can occur in the brain or spine and varies in frequency between adults and children depending on the molecular subtype [35] [36]. Grade III ependymomas occur most often in the brain but also the spine [36].
The location and molecular features of each ependymoma subtype can provide more accurate prognostic information than grade alone. According to the 2021 World Health Organization (WHO) classification, the main subtypes are supratentorial ependymoma (ZFTA fusion-positive and YAP1 fusion-positive), posterior fossa group A (PFA) ependymoma, posterior fossa group B (PFB) ependymoma, spinal ependymoma, spinal ependymoma with MYCN amplification, myxopapillary ependymoma, and subependymoma [35]. On magnetic resonance imaging (MRI), ependymomas appear as one or more well-defined masses that often enhance with contrast, making them appear brighter on the scan [36].
The exact cause of ependymomas remains unclear. Researchers have not identified the specific mutations causing ependymomas, but they do occur in individuals with certain genetic conditions, such as Neurofibromatosis Type 2 [37]. Ependymomas can form anywhere within the CNS but often develop near the ventricles of the brain and the central canal of the spinal cord. Rarely, they may form outside the CNS, such as in the ovaries [38]. These tumors originate from radial glial cells, one of the three types of glial cells that support the CNS [37[38].
Ependymomas can spread outside the CNS; however, they can disseminate to other areas within the CNS through cerebrospinal fluid (CSF) [37][38]. Symptoms depend on the tumor’s location. Brain ependymomas may cause headaches, nausea and vomiting, and dizziness. Spinal ependymomas may result in back pain, numbness and weakness in the arms, legs, or trunk, and sexual dysfunction or urinary and bowel problems [35].
Ependymomas affect both children and adults [39]. Tumors located in the lower part of the brain (posterior fossa) are more common in children, while spinal ependymomas are more prevalent in adults [37] [38[39]. They occur more often in males than females and are most common among non-Hispanic white individuals [39]. Almost 20,000 people in the United States are living with this type of tumor [35].

Self Quiz
Ask yourself...
- How might the distinct locations of ependymomas, such as in the posterior fossa for children and the spine for adults, influence the symptoms and treatment approach for each age group?
- Why could the molecular features and subtypes of ependymomas, as identified in the WHO classification, provide better prognostic information than grade alone, and how might this impact treatment planning?
- Considering that ependymoma can spread within the CNS through cerebrospinal fluid (CSF), what challenges does this pose for treatment and managing potential recurrence?
Glioblastoma Multiforme (GBM)
Glioblastoma is the most common malignant brain tumor, accounting for 47.7% of all brain and central nervous system (CNS) tumors [4] 40]. The incidence rate is 3.21 per 100,000 individuals, with a median diagnosis age of 64 years [4][40]. It is more prevalent in men than women [4][40]. The prognosis remains poor, with 40% of patients surviving the first-year post-diagnosis, and only 17% surviving into the second year [4][40].
Risk factors associated with GBM include prior therapeutic radiation, decreased susceptibility to allergies, impaired immune response, and certain hereditary cancer syndromes such as Li-Fraumeni syndrome and Lynch syndrome [41][42]. Symptoms vary depending on the tumor’s location but may include persistent headaches, double or blurred vision, vomiting, loss of appetite, mood and personality changes, cognitive difficulties, new-onset seizures, and gradual onset of speech difficulties [42].
Glioblastoma multiforme (GBM), also known as grade IV astrocytoma, is the most aggressive and invasive form of glioma—a type of tumor arising from glial cells in the brain [4][42]. Characterized by rapid growth and extensive invasion into nearby brain tissue, GBM carries a poor prognosis [4][42]. Glioblastomas (GBMs) can develop either as primary tumors (de novo) or progress from lower grade astrocytomas, occurring in the cerebral hemispheres, especially in the frontal and temporal lobes [4][42].
Treatment of GBM presents several challenges due to its localization within the brain, inherent resistance to conventional therapies, and the limited regenerative capacity of brain tissue. Malignant cells tend to infiltrate adjacent brain areas, and the tumor’s disrupted blood supply can inhibit effective drug delivery. In addition, tumor capillary leakage often leads to peritumoral edema and intracranial hypertension [48]. Patients may also experience tumor-induced seizures and neurotoxicity from treatments directed at gliomas [48].
Diagnosis involves sophisticated imaging techniques to determine the tumor’s location. Providers use computed tomography (CT) scans and magnetic resonance imaging (MRI) as diagnostic tools. Intraoperative MRI can assist during surgery for tissue biopsies and tumor removal, while magnetic resonance spectroscopy (MRS) examines the tumor’s chemical profile. Conventional MRI is essential for studying astrocytomas; higher-grade tumors enhance with contrast and may show central necrosis [43]. MRI spectroscopy provides information on the tumor’s chemical composition, aiding in non-invasive tissue sampling, though it is not as definitive as a biopsy [44]. Functional MRI (fMRI) identifies brain regions activated during specific tasks, which is crucial for surgical planning to avoid critical areas responsible for speech, motor function, or vision [44].
After detection, a neurosurgeon obtains tumor tissue for biopsy, and a neuropathologist examines it to assign a name and grade based on the World Health Organization (WHO) classification. Histological grading assesses features such as cytologic atypia, anaplasia, mitotic activity, microvascular proliferation, and necrosis [27]. Grade II tumors exhibit cytologic atypia with variations in nuclear shape, size, and hyperchromasia [4][45]. Grade III tumors show anaplasia and increased mitotic activity, indicating higher cellularity [4][45]. Grade IV tumors, like GBM, demonstrate microvascular proliferation and necrosis, signifying the most aggressive behavior [4][45].

Self Quiz
Ask yourself...
- Given the aggressive nature and poor prognosis of glioblastoma multiforme (GBM), what factors might limit the effectiveness of conventional treatments, and how might these influence treatment outcomes?
- How do the specific risk factors, such as prior radiation and hereditary cancer syndromes, contribute to the development of GBM, and what does this imply for potential prevention or early detection strategies?
- In what ways does the complex diagnostic process for GBM, involving advanced imaging and histological grading, aid in treatment planning, and why is precise grading crucial for managing such an aggressive tumor?
Other Types of Malignant Brain Tumors
- Medulloblastomas: These tumors develop in the cerebellum and affect children. Although they are high-grade, they respond well to radiation and chemotherapy.
- Oligodendrogliomas: Derived from the cells responsible for producing myelin, the protective sheath around brain nerves. These tumors can vary in aggressiveness.
- Hemangioblastomas: Slow-growing tumors that often occur in the cerebellum. Originating from blood vessels, they can become large and are associated with cyst formation. Hemangioblastomas are most common in adults aged 40 to 60, and men more often develop them than women [4].
- Rhabdoid Tumors: These are rare, aggressive tumors that can spread throughout the central nervous system. They can also occur in other parts of the body, such as the kidneys. Rhabdoid tumors are more common in young children but can also occur in adults [4].

Self Quiz
Ask yourself...
- How might the origin and cellular characteristics of different malignant brain tumors, such as medulloblastomas in children versus hemangioblastomas in adults, influence their treatment responses and prognosis?
- In what ways do the unique growth patterns and spread potential of aggressive tumors like rhabdoid tumors impact the challenges in treatment and long-term management, particularly in younger patients?
Pediatric Brain Tumors
Pediatric brain tumors differ from those seen in adults, as they often arise from different tissues. Treatments that may be well-tolerated by adult brains, such as radiation therapy, can hinder normal brain development in children younger than five years old [4] [10]. These co-factors approach to treating pediatric brain tumors is more complex and nuanced.
The Pediatric Brain Tumor Foundation reports that almost 4,200 children in the U.S. receive a brain tumor diagnosis each year, and 72% of those affected are younger than 15 years old [4] [46]. The majority of pediatric brain tumors develop in the posterior fossa, the region at the back of the brain [47]. Children with brain tumors present with symptoms of hydrocephalus (fluid buildup in the brain) or neurological deficits, such as impaired movement in the face or body.
Certain types of brain tumors are more common in children than adults. The most frequent pediatric brain tumors include:
- Medulloblastomas
- Low-grade astrocytomas (pilocytic astrocytomas)
- Ependymomas
- Craniopharyngiomas
- Brainstem gliomas
Each type requires specialized treatment approaches, tailored to the child’s age and the tumor’s location and characteristics, to preserve as much normal brain function and development as possible.

Self Quiz
Ask yourself...
- How does the need to preserve normal brain development in young children influence the choice and intensity of treatments for pediatric brain tumors compared to treatments for adults?
- In what ways might the location of pediatric brain tumors, such as in the posterior fossa, contribute to specific symptoms and treatment challenges that are less common in adult cases?
- How might the diverse types of pediatric brain tumors, like medulloblastomas or brainstem gliomas, require unique treatment approaches, and what role does the child’s age play in these decisions?
Incidence of Malignant Brain Tumors in Adults
The incidence rate of all primary malignant and non-malignant brain and other central nervous system (CNS) tumors in the United States stands at 24.83 cases per 100,000 people, amounting to 453,623 cases overall [49]. This includes a rate of 6.94 per 100,000 for malignant tumors (126,729 cases) and 17.88 per 100,000 for non-malignant tumors (326,894 cases) [49].
Incidence rates are higher in females (27.85 per 100,000) compared to males (21.62 per 100,000) [49]. In 2023, experts expect 94,390 new cases of primary brain and other CNS tumors, comprising 26,940 malignant and 67,440 non-malignant cases [49].
In the United States, the incidence and mortality rates of primary brain and other central nervous system (CNS) tumors vary by age group. Among children aged 0-14, the incidence rate is 5.44 cases per 100,000 people, totaling 17,136 cases over five years, with an annual average of 3,427 new cases. Experts estimate 3,920 new cases in this group for 2023 [49].
In adolescents aged 15-19, the incidence rate rises to 7.51 per 100,000, contributing to a five-year total of 7,863 cases, averaging 1,573 per year. Within this age range, 2,679 cases are malignant, with an incidence rate of 2.56 per 100,000, while 5,168 are non-malignant, at 4.95 per 100,000. Experts project around 1,310 new adolescent cases for 2023. Brain and CNS tumors among adolescents accounted for about 2% of all cases reported from 2015 to 2019 [49].
The adolescent and young adult (AYA) population, ages 15-39, has an incidence rate of 12.00 per 100,000, yielding a five-year total of 64,238 cases [49]. Non-malignant tumors occur more frequently in this group, with an incidence rate of 8.79 per 100,000 compared to 3.21 for malignant tumors. Experts project 11,660 new AYA cases in 2023 [49]. Among adults aged 40 and above, the incidence rate reaches 44.97 per 100,000, totaling 372,249 cases over five years [49]. Non-malignant tumors (33.33 per 100,000) occur more than malignant ones (11.64 per 100,000). Experts project 79,340 new cases in this age group for 2023 [49].
The average annual mortality rate for primary malignant brain and CNS tumors from 2016 to 2020 was 4.42 per 100,000, resulting in 86,030 deaths [49]. This included a higher rate in males (5.40 per 100,000) than in females (3.57 per 100,000), with an annual average of 17,206 deaths [49]. Lifetime risk estimates show that an individual in the U.S. faces a 0.6% chance of developing a primary malignant brain or CNS tumor and a 0.50% chance of dying from it [49] [50].
Males have an elevated lifetime risk of developing such tumors at 0.70% and a 0.56% chance of dying from them, compared to females, who have a 0.54% risk of diagnosis and a 0.42% risk of mortality [49].

Self Quiz
Ask yourself...
- How might the differences in incidence and mortality rates of brain and CNS tumors between age groups, such as adolescents versus adults over 40, affect screening practices and healthcare resources?
- In what ways do the higher incidence rates of primary brain and CNS tumors in females and the elevated mortality risk in males suggest gender-specific factors influencing brain tumor development and survival outcomes?
- Considering the overall lifetime risk for developing a malignant brain tumor, what preventative strategies or early detection methods could reduce these rates, especially among higher-risk populations?
Brain Tumor Causes
Brain tumors arise when damage occurs to specific genes on a cell’s chromosomes, causing them to malfunction [1]. These genes regulate the rate at which the cell divides (if it divides at all) and repair genes that fix defects of other genes, as well as genes that could cause the cell to self-destruct if the damage is beyond repair. In some cases, an individual may be born with partial defects in one or more of these genes. Environmental factors may then lead to further damage. In other cases, the environmental injury to the genes may be the only cause. It is not known why some people in an “environment” develop brain tumors, while others do not.
Once a cell is dividing, internal mechanisms to check its growth are damaged, the cell can grow into a tumor. Another line of defense may be the body’s immune system, which could detect the abnormal cell and kill it. Tumors may produce substances that block the immune system from recognizing the abnormal tumor cells and overpower all internal and external deterrents to its growth.
A growing tumor demands more oxygen and nutrients than the local blood supply, which is meant for normal tissue, can provide [51]. Tumors can produce substances called angiogenesis factors that promote the growth of blood vessels [51]. The new vessels that grow increase the supply of nutrients to the tumor, and the tumor becomes dependent on these new vessels. Researchers study this area, but they must expand their efforts to translate this knowledge into potential therapies.

Self Quiz
Ask yourself...
- How might genetic predispositions and environmental factors interact in ways that increase an individual’s risk of developing a brain tumor, and why might some people in similar environments remain unaffected?
- In what ways does the ability of a tumor to evade the immune system and promote its own blood supply through angiogenesis complicate treatment, and how could targeting these processes improve therapeutic outcomes?
Testing and Diagnosis of Brain Tumors
The diagnosis of brain tumors relies on advanced imaging techniques and specialized tests to identify the presence, location, and characteristics of the tumor. A combination of imaging modalities and, in some cases, biopsy is essential to establish a precise diagnosis.
Key diagnostic tools include:
- Computed Tomography (CT) or Computed Axial Tomography (CAT) Scan: CT scans provide detailed cross-sectional images of the brain using X-rays. These scans can detect abnormalities such as masses, bleeding, and swelling, helping to identify the presence of a brain tumor.
- Magnetic Resonance Imaging (MRI): MRI is a powerful imaging technique that uses magnetic fields and radio waves to generate detailed images of brain tissue. MRI is often the gold standard for diagnosing brain tumors due to its superior resolution compared to CT scans.
- Intraoperative MRI: Surgeons use intraoperative MRI during brain tumor surgery to guide tissue biopsies and assist in tumor removal. This real-time imaging allows the neurosurgeon to confirm the extent of the tumor resection while minimizing damage to surrounding healthy tissue.
- Magnetic Resonance Spectroscopy (MRS): MRS is a non-invasive imaging technique that examines the chemical composition of a tumor, providing additional information about its nature. MRS can help differentiate between several types of brain lesions, including tumors, infections, and inflammation, based on their metabolic profiles.
- Positron Emission Tomography (PET) Scan: PET scans use a radioactive tracer to assess metabolic activity in brain tissues. This technique is useful in detecting recurrent brain tumors and differentiating between tumor growth and treatment-related changes, such as radiation necrosis.
Biopsy
In many cases, a definitive diagnosis requires a biopsy, where the surgeon removes a small tumor sample and examines it under a microscope. A neurosurgeon performs the biopsy, and a pathologist analyzes the tissue sample to determine if the tumor is benign or malignant. The pathologist also assigns a grade to the tumor, which helps guide treatment decisions based on the tumor’s aggressiveness.
- Stereotactic Biopsy: This less invasive procedure uses imaging guidance (MRI or CT) to target and remove a small piece of the tumor for examination [4]. Surgeons often perform this procedure when the tumor lies in a difficult-to-reach area or when a full resection is not feasible. The combination of imaging techniques and biopsy provides a comprehensive understanding of the tumor’s characteristics, allowing for more tailored treatment planning.

Self Quiz
Ask yourself...
- How does the information gained from a biopsy, including tumor grade and location, influence treatment planning and the choice between more invasive procedures and targeted approaches like a stereotactic biopsy?
Brain Tumor Treatment and Care
Treatment of brain tumors, whether primary or metastatic, benign, or malignant, involves a combination of surgery, radiation therapy, and/or chemotherapy. Clinicians tailor treatment choices to each patient based on factors like tumor location, size, type, and the patient’s overall health. Radiation and chemotherapy often treat malignant, residual, or recurrent tumors, but each therapy carries potential risks and side effects that require careful consideration.
Surgery
Surgical removal of the tumor is often the first-line treatment, as complete or near-complete resection can improve outcomes. The goal of surgery is the preservation of essential neurological functions such as speech, mobility, and cognition [56]. In some cases, a drain (external ventricular drain, or EVD) relieves fluid buildup in the brain during recovery.
- Surgical Navigation Systems: Introduced in the 1990s, these computerized systems use pre-operative imaging to guide the neurosurgeon during tumor resection. They enhance precision and reduce risk, making it possible to remove previously inoperable tumors. However, they rely on pre-surgical scans and cannot account for brain movements during surgery. Researchers develop new techniques using intraoperative MRI and ultrasound to overcome this limitation.
- Intraoperative Language Mapping: For tumors affecting language areas of the brain, a patient may remain awake during surgery. Surgeons map the patient’s language function during the operation to identify tumor areas they can remove without impairing speech or comprehension. This technique is useful for large, dominant-hemisphere gliomas.
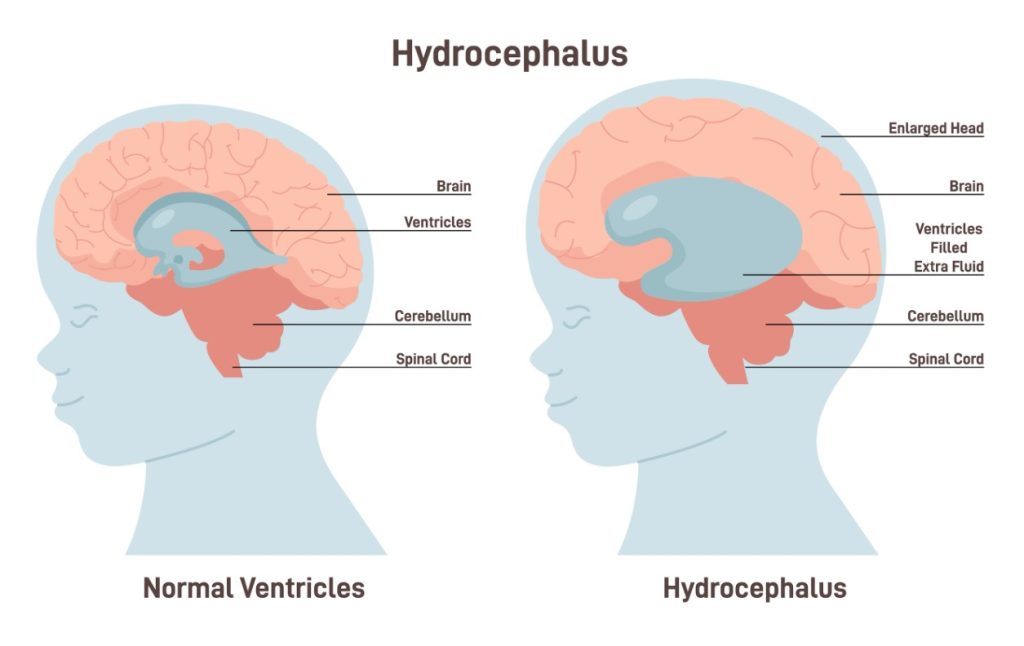
- Ventriculoperitoneal Shunting: Some patients may develop hydrocephalus, a condition where cerebrospinal fluid (CSF) accumulates in the brain, causing increased pressure [4]. To relieve this pressure, a shunt diverts the fluid to the peritoneal cavity in the abdomen. If the shunt becomes blocked, symptoms such as headaches, vomiting, and confusion may occur [4].
- Endoscopic Third Ventriculostomy (ETV): In some cases, surgeons perform an ETV to bypass fluid obstructions, eliminating the need for a permanent shunt [53].

Self Quiz
Ask yourself...
- How do factors like tumor location, patient health, and specific neurological functions influence the decision to use various surgical approaches, such as intraoperative language mapping or surgical navigation systems?
- In what ways might the potential side effects and risks of treatments like radiation and chemotherapy affect the overall treatment strategy, especially in cases where preserving neurological function is essential?
Radiation Therapy
Radiation therapy uses high-energy X-rays to destroy cancer cells and shrink tumors [52].
Standard External Beam Radiotherapy uses targeted radiation beams to treat the tumor while sparing surrounding healthy tissue. Newer delivery methods, such as intensity-modulated radiotherapy (IMRT), allow for even more precise targeting and reduce the risk of long-term radiation damage [54].
- Proton Beam Therapy: A type of radiation therapy that uses protons to deliver radiation to the tumor with minimal impact on nearby healthy tissues.
- Stereotactic Radiosurgery (SRS): Techniques such as Gamma Knife, CyberKnife, and Novalis deliver focused radiation beams to the tumor from multiple angles, minimizing damage to surrounding tissues [55].

Self Quiz
Ask yourself...
- How might the choice between distinct types of radiation therapies, such as IMRT, proton beam therapy, and stereotactic radiosurgery, depend on factors like tumor size, location, and the need to minimize damage to surrounding healthy tissues?
Chemotherapy
Physicians often use chemotherapy to treat certain pediatric tumors, lymphomas, and some oligodendrogliomas. For the most malignant primary brain tumors, chemotherapy can improve survival in 20% of patients, although predicting who will benefit is challenging [4]. Some physicians may opt not to use chemotherapy due to its potential side effects, which can include nausea, immune system suppression, and lung scarring.
Chemotherapy works by damaging cancer cells, which are less capable of repairing themselves than normal cells [57]. However, resistance to chemotherapy can occur when tumor cells survive treatment or when the drugs cannot penetrate the brain due to the blood-brain barrier [57]. Researchers are exploring methods to improve drug delivery, such as disrupting the blood-brain barrier or injecting chemotherapy directly into the tumor.
Chemotherapy-Imbued Wafers: The FDA approved these wafers in 1996. Surgeons implant them during procedures, allowing the wafers to release chemotherapy drugs into the tumor site, which reduces systemic side effects [58].

Self Quiz
Ask yourself...
- What challenges do factors like the blood-brain barrier and chemotherapy resistance present in treating brain tumors, and how might innovations like chemotherapy-imbued wafers address these issues?
Laser Interstitial Thermal Therapy (LITT)
LITT is a newer, less invasive technique used to treat smaller brain tumors in hard-to-reach areas. Providers insert a catheter into the tumor and use laser energy to destroy the tumor tissue. While promising, the long-term efficacy of this treatment is still under investigation [59].
Investigational Therapies
Researchers are studying emerging therapies for tumors with poor prognoses under conventional treatment. These investigational treatments include:
- Immunotherapy: Aims to enhance the body’s immune system to better recognize and attack tumor cells.
- Anti-Angiogenesis Therapy: Seeks to block the formation of new blood vessels that tumors need to grow.
- Gene Therapy: Attempts to repair or modify the genes involved in tumor growth.
- Targeted Toxin Therapy: Involves attaching toxins to molecules that specifically target tumor cells, sparing normal tissue.

Self Quiz
Ask yourself...
- How might investigational therapies like LITT, immunotherapy, and gene therapy improve outcomes for patients with hard-to-treat brain tumors, and what factors would determine their suitability compared to conventional treatments?
Comprehensive Care
Malignant brain tumors pose complex challenges due to their aggressive nature and the intricate involvement of central nervous system structures. Originating from genetic mutations that disrupt normal cell growth, these tumors vary in type and location, with primary malignant tumors often forming in the brain and secondary tumors spreading from other body regions. The World Health Organization (WHO) tumor grading system, which ranges from grade 1 to 4, assists clinicians in assessing tumor aggressiveness and selecting appropriate treatment approaches [13]. Common malignant brain tumors include glioblastomas, astrocytomas, oligodendrogliomas, and ependymomas, each presenting unique characteristics and requiring targeted therapeutic interventions [5]. Symptoms often vary based on tumor size and location, ranging from headaches and seizures to personality changes and cognitive deficits [6].
Advancements in treatment, including immunotherapy, gene therapy, and precision-targeted radiation, offer new hope, with tools like intraoperative MRI and functional mapping enhancing surgical precision and preserving neurological function [4][30]. Continued research and an understanding of molecular pathways driving these tumors are essential to improving diagnosis, treatment options, and survival outcomes for individuals affected by malignant brain tumors.
Successful brain tumor treatment requires a multidisciplinary approach, involving neurosurgeons, oncologists, radiologists, and other specialists. The goal is to tailor treatment to the individual’s specific tumor characteristics while minimizing damage to normal brain tissue and preserving quality of life. Continuous advancements in technology and research are paving the way for more effective and less invasive treatment options.

Self Quiz
Ask yourself...
- How does the multidisciplinary approach to treating malignant brain tumors benefit patient outcomes, and what roles do different specialists play in balancing effective treatment with quality of life?
- In what ways might advancements in tools like intraoperative MRI and precision-targeted therapies impact the long-term management and survival of patients with aggressive brain tumors?
References + Disclaimer
- Brain and spinal cord tumors. (2024). National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/health-information/disorders/brain-and-spinal-cord-tumors
- Grades of brain tumours. (2024). https://www.cancerresearchuk.org/about-cancer/brain-tumours/grades/
- Tumor grade. (2022, August 1). Cancer.gov. https://www.cancer.gov/about-cancer/diagnosis-staging/diagnosis/tumor-grade
- American Association of Neurological Surgeons. (2024, June 25). Brain Tumors – AANS. AANS. https://www.aans.org/patients/conditions-treatments/brain-tumors/
- Brain tumors and brain cancer. (2024). Johns Hopkins Medicine. https://www.hopkinsmedicine.org/health/conditions-and-diseases/brain-tumor
- NHS informs. (2024, October 10). Malignant brain tumour (cancerous) | NHS inform. NHS Inform. https://www.nhsinform.scot/illnesses-and-conditions/cancer/cancer-types-in-adults/malignant-brain-tumour-cancerous/
- Lucifero, A. G., Luzzi, S., Brambilla, I., Trabatti, C., Mosconi, M., Savasta, S., & Foiadelli, T. (2020). Innovative therapies for malignant brain tumors: the road to a tailored cure. Acta Bio-medica: Atenei Parmensis, 91, 5–17. https://doi.org/10.23750/abm.v91i7-s.9951
- A new vaccine to target Treatment-Resistant glioblastoma. (2024). Center for Cancer Research. https://ccr.cancer.gov/neuro-oncology-branch/a-new-vaccine-to-target-treatment-resistant-glioblastoma
- Nasrallah, M. P., Zhao, J., Tsai, C. C., Meredith, D., Marostica, E., Ligon, K. L., Golden, J. A., & Yu, K. (2023). Machine learning for cryosection pathology predicts the 2021 WHO classification of glioma. Med, 4(8), 526-540.e4. https://doi.org/10.1016/j.medj.2023.06.002
- Brain cancer (Brain tumor). (2024, September 9). Cleveland Clinic. https://my.clevelandclinic.org/health/diseases/6149-brain-cancer-brain-tumor
- Amsbaugh, M. J., & Kim, C. S. (2023, April 3). Brain metastasis. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK470246/
- Brain metastases. (2024). MD Anderson Cancer Center. https://www.mdanderson.org/cancer-types/brain-metastases.html
- Torp, S. H., Solheim, O., & Skjulsvik, A. J. (2022). The WHO 2021 Classification of Central Nervous System tumours: a practical update on what neurosurgeons need to know—a minireview. Acta Neurochirurgica, 164(9), 2453–2464. https://doi.org/10.1007/s00701-022-05301-y
- Mesfin, F. B., Karsonovich, T., & Al-Dhahir, M. A. (2024, August 12). Gliomas. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK441874/
- Signs & Symptoms – National Brain Tumor Society. (2024, September 13). National Brain Tumor Society. https://braintumor.org/brain-tumors/diagnosis-treatment/signs-symptoms/
- Young, J. S., Al-Adli, N., Sibih, Y. E., Scotford, K. L., Casey, M., James, S., & Berger, M. S. (2022). Recognizing the psychological impact of a glioma diagnosis on mental and behavioral health: a systematic review of what neurosurgeons need to know. Journal of Neurosurgery, 139(1), 11–19. https://doi.org/10.3171/2022.9.jns221139
- Meyer, J., Yu, K., Luna-Figueroa, E., Deneen, B., & Noebels, J. (2024). Glioblastoma disrupts cortical network activity at multiple spatial and temporal scales. Nature Communications, 15(1). https://doi.org/10.1038/s41467-024-48757-5
- Kapoor, M., & Gupta, V. (2024, May 28). Astrocytoma. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK559042/
- Wei, D. C., & Morrison, E. H. (2023, May 1). Histology, astrocytes. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK545142/
- Çınar, O. E., Göker, H., Fidan, K., Aydın, Ö., Pashayev, T., Malkan, Ü. Y., Velet, M., Büyükaşık, Y., Aksu, S., Özcebe, O. İ., Haznedaroğlu, İ. C., Sayınalp, N., Vural, F., Turgut, M., Ünal, A., & Demiroğlu, H. (2022). Prophylactic Central Nervous System Irradiation Is Not Indispensable in Adult Patients with Acute Lymphoblastic Leukemia: A Multicenter Retrospective Cohort Study. Turkish Journal of Hematology, 39(3), 152–159. https://doi.org/10.4274/tjh.galenos.2022.2021.0680
- Children with ALL Can Skip Brain Radiation. (2019, November 25). Cancer.gov. https://www.cancer.gov/news-events/cancer-currents-blog/2019/childhood-all-relapse-brain-radiation
- Hamblin, R., Vardon, A., Akpalu, J., Tampourlou, M., Spiliotis, I., Sbardella, E., Lynch, J., Shankaran, V., Mavilakandy, A., Gagliardi, I., Meade, S., Hobbs, C., Cameron, A., Levy, M. J., Ayuk, J., Grossman, A., Ambrosio, M. R., Zatelli, M. C., Reddy, N., . . . Karavitaki, N. (2022). Risk of second brain tumour after radiotherapy for pituitary adenoma or craniopharyngioma: a retrospective, multicentre, cohort study of 3679 patients with long-term imaging surveillance. The Lancet Diabetes & Endocrinology, 10(8), 581–588. https://doi.org/10.1016/s2213-8587(22)00160-7
- Cell phones and cancer Risk fact sheet. (2024, April 4). Cancer.gov. https://www.cancer.gov/about-cancer/causes-prevention/risk/radiation/cell-phones-fact-sheet
- Schneider, K., Zelley, K., Nichols, K. E., Levine, A. S., & Garber, J. (2024, September 5). Li-Fraumeni Syndrome. GeneReviews® – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK1311/
- National Center for Biotechnology Information (US). (1998). The p53 tumor suppressor protein. Genes and Disease – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK22268/
- Hirtz, A., Rech, F., Dubois-Pot-Schneider, H., & Dumond, H. (2020). Astrocytoma: A Hormone-Sensitive tumor? International Journal of Molecular Sciences, 21(23), 9114. https://doi.org/10.3390/ijms21239114
- Aiman, W., Gasalberti, D. P., & Rayi, A. (2023, May 6). Low-Grade Gliomas. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK560668/
- Malik, J. R., Podany, A. T., Khan, P., Shaffer, C. L., Siddiqui, J. A., Baranowska‐Kortylewicz, J., Le, J., Fletcher, C. V., Ether, S. A., & Avedissian, S. N. (2023). Chemotherapy in pediatric brain tumor and the challenge of the blood–brain barrier. Cancer Medicine, 12(23), 21075–21096. https://doi.org/10.1002/cam4.6647
- Zhang, X., Ma, H., Gao, Y., Liang, Y., Du, Y., Hao, S., & Ni, T. (2024). The tumor microenvironment: signal transduction. Biomolecules, 14(4), 438. https://doi.org/10.3390/biom14040438
- Oligodendroglioma and other IDH-Mutated Tumors: Diagnosis and Treatment. (2024, August 20). Cancer.gov. https://www.cancer.gov/rare-brain-spine-tumor/tumors/oligodendroglioma
- OncologyPRO. (2016, November 20). 1p/19q Co-deletion in Glioma: ESMO Biomarker Factsheet. OncologyPRO. https://oncologypro.esmo.org/education-library/factsheets-on-biomarkers/1p-19q-co-deletion-in-glioma
- The genetics of cancer. (2024, August 8). Cancer.gov. https://www.cancer.gov/about-cancer/causes-prevention/genetics
- Karaman, A. K., Korkmazer, B., Urganci, N., Baş, G., Arslan, S., Comunoglu, N., Hanci, M. M., & Kızılkılıç, O. (2022). Case report: Spinal drop metastasis of IDH-mutant, 1p/19q-codeleted oligodendroglioma. Frontiers in Neurology, 13. https://doi.org/10.3389/fneur.2022.1086591
- Ostrom, Q. T., Cote, D. J., Ascha, M., Kruchko, C., & Barnholtz-Sloan, J. S. (2018). Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncology, 4(9), 1254. https://doi.org/10.1001/jamaoncol.2018.1789
- Ependymoma: diagnosis and treatment. (2024, August 20). Cancer.gov. https://www.cancer.gov/rare-brain-spine-tumor/tumors/ependymoma
- Seo, S., Paul, S. K., Shikder, M., Khanam, M., Ghosh, P., Hasib, T. A., Ahmed, K. A., Sikdar, S., Uddin, M. J., & Kwon, Y. (2021). An Insight into Pathophysiological Features and Therapeutic Advances on Ependymoma. Cancers, 13(13), 3221. https://doi.org/10.3390/cancers13133221
- Yao, Y., Mack, S. C., & Taylor, M. D. (2011). Molecular genetics of ependymoma. Chinese Journal of Cancer, 30(10), 669–681. https://doi.org/10.5732/cjc.011.10129
- Zamora, E. A., & Alkherayf, F. (2023, June 26). Ependymoma. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK538244/
- Childhood ependymoma. (2024, June 5). Cancer.gov. https://www.cancer.gov/types/brain/patient/childhood-ependymoma
- Styliara, E. I., Astrakas, L. G., Alexiou, G., Xydis, V. G., Zikou, A., Kafritsas, G., Voulgaris, S., & Argyropoulou, M. I. (2024). Survival Outcome Prediction in Glioblastoma: Insights from MRI Radiomics. Current Oncology, 31(4), 2233–2243. https://doi.org/10.3390/curroncol31040165
- Glioblastoma – Symptoms and causes. (2024). Mayo Clinic. https://www.mayoclinic.org/diseases-conditions/glioblastoma/symptoms-causes/syc-20569077
- Kanderi, T., Munakomi, S., & Gupta, V. (2024, May 6). Glioblastoma multiforme. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK558954/
- Laino, M. E., Young, R., Beal, K., Haque, S., Mazaheri, Y., Corrias, G., Bitencourt, A. G., Karimi, S., & Thakur, S. B. (2020). Magnetic resonance spectroscopic imaging in gliomas: clinical diagnosis and radiotherapy planning. BJR|Open, 2(1), 20190026. https://doi.org/10.1259/bjro.20190026
- Lakhani, D. A., Sabsevitz, D. S., Chaichana, K. L., Quiñones-Hinojosa, A., & Middlebrooks, E. H. (2023). Current state of functional MRI in the presurgical planning of brain tumors. Radiology Imaging Cancer, 5(6). https://doi.org/10.1148/rycan.230078
- Mesfin, F. B., Karsonovich, T., & Al-Dhahir, M. A. (2024, August 12). Gliomas. StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK441874/
- Ostrom, Q. T., Price, M., Ryan, K., Edelson, J., Neff, C., Cioffi, G., Waite, K. A., Kruchko, C., & Barnholtz-Sloan, J. S. (2022). CBTRUS Statistical Report: Pediatric Brain Tumor Foundation childhood and adolescent primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro-Oncology, 24(Supplement_3), iii1–iii38. https://doi.org/10.1093/neuonc/noac161
- American Childhood Cancer Organization. (2020, April 1). Brain Tumors in Children – ACCO. ACCO. https://www.acco.org/blog/brain-tumors-in-children/?gad_source=1
- Ohmura, K., Tomita, H., & Hara, A. (2023). Peritumoral edema in Gliomas: A Review of Mechanisms and Management. Biomedicines, 11(10), 2731. https://doi.org/10.3390/biomedicines11102731
- CBTRUS Fact Sheet 2023 – CBTRUS. (2023, October 8). CBTRUS. https://cbtrus.org/cbtrus-fact-sheet/
- Lifetime risk of developing or dying from cancer. (2024). American Cancer Society. https://www.cancer.org/cancer/risk-prevention/understanding-cancer-risk/lifetime-probability-of-developing-or-dying-from-cancer.html
- Angiogenesis inhibitors. (2018, April 2). Cancer.gov. https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/angiogenesis-inhibitors-fact-sheet
- Radiation therapy for cancer. (2019, January 8). Cancer.gov. https://www.cancer.gov/about-cancer/treatment/types/radiation-therapy
- Surgery to remove fluid in the brain. (2024). https://www.cancerresearchuk.org/about-cancer/brain-tumours/treatment/surgery/remove-fluid
- Intensity-modulated radiation therapy (IMRT) – Mayo Clinic. (2024). https://www.mayoclinic.org/tests-procedures/intensity-modulated-radiation-therapy/about/pac-20385147
- Hynes, P. R., & Das, J. M. (2023, July 25). Stereotactic radiosurgery (SRS) and stereotactic body radiotherapy (SBRT). StatPearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK542166/
- Parsons, M. W., Peters, K. B., Floyd, S. R., Brown, P., & Wefel, J. S. (2021). Preservation of neurocognitive function in the treatment of brain metastases. Neuro-Oncology Advances, 3(Supplement_5), v96–v107. https://doi.org/10.1093/noajnl/vdab122
- How chemotherapy works. (2024). Cancer Research UK. https://www.cancerresearchuk.org/about-cancer/treatment/chemotherapy/how-chemotherapy-works
- Brownlee, C. (2020, March 31). New research: Electrospinning to deliver multiple chemotherapy drugs to the brain. Johns Hopkins Medicine. https://www.hopkinsmedicine.org/news/articles/2020/03/new-research-electrospinning-to-deliver-multiple-chemotherapy-drugs-to-the-brain
- Chen, C., Lee, I., Tatsui, C., Elder, T., & Sloan, A. E. (2021). Laser interstitial thermotherapy (LITT) for the treatment of tumors of the brain and spine: a brief review. Journal of Neuro-Oncology, 151(3), 429–442. https://doi.org/10.1007/s11060-020-03652-z
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click the Green MARK COMPLETE Button Below
To receive your certificate
