Course
Medical Cannabis in Epilepsy Treatment
Course Highlights
- In this Medical Cannabis in Epilepsy Treatment course, we will learn about the evolving landscape of Cannabis therapeutics.
- You’ll also learn the legal and ethical considerations of Cannabis within the treatment of Epilepsy.
- You’ll leave this course with a broader understanding of robust strategies for patient education, emphasizing the need for accurate, evidence-based information to help patients make informed choices about their treatment options.
About
Contact Hours Awarded: 3.5
Course By:
R.E. Hengsterman
MSN, RN, MA
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction
Medical treatments advance on a continuum, offering new treatment methodologies and opportunities for healthcare professionals. In recent years Cannabis sativa has garnered significant attention.
Despite its classification as a Schedule I Substance under the Controlled Substances Act, growing research suggests that cannabis may offer therapeutic benefits in treating various medical conditions including pain, spasticity, nausea, posttraumatic stress disorder (PTSD), cancer, cachexia, glaucoma, HIV/AIDS, degenerative neurological conditions, and epilepsy (1).
However, there is debate regarding the use of cannabis and its legal status varies across states. This creates a complicated regulatory environment for medical professionals, including nurses. This course aims to equip nurses with the essential knowledge needed to navigate the complexities of cannabis in the treatment of epilepsy.

Self Quiz
Ask yourself...
- How can nurses reconcile the existing disparities between federal legislation, state-specific regulations, and emerging scientific evidence to provide ethical, evidence-based care to their patients?
Brief Overview of Epilepsy
Epilepsy is not a single specific disease, or isolated syndrome, but a broad category of disordered brain functions affecting approximately 1% of the general population. The existence of seizures, descriptions of seizure-like activity, and its terminology dates to the 25th century – 2500 BC (2).
The mischaracterization and stigmatization of epilepsy has occurred throughout history. It was once linked to mystical, supernatural, demonic, and religious occurrences. In ancient Greece, Hippocrates took a controversial stance on epilepsy and related conditions termed “Sacred Disease.” He dismissed the religious origin of illness, including epilepsy, and shifted the perspective from the mystical and supernatural to a scientific approach grounded in rational thought (2).
Our contemporary understanding of epilepsy as a complex neurological disorder characterized by seizures arises from the systemic analysis of anatomy and physiology by British neurologist John Hughlings-Jackson (3). Hughlings-Jackson provided a systematic framework for diagnosing diseases of the nervous system and guided clinical practice to a more nuanced, evidence-based approach to the treatment of epilepsy.
The standardized classification and terminology established by the International League Against Epilepsy (ILAE) in 1981 for “epileptic seizures,” and in 1989 for “epilepsies and epileptic syndromes,” serve as a basis for categorizing and distinguishing various forms of epilepsy (4).
There is currently no cure for epilepsy, only symptomatic pharmacological treatment.

Self Quiz
Ask yourself...
- How might the historical stigmatization of epilepsy impact contemporary patient care and public perception of the condition?
- Can you name examples of limitations of pharmacological treatments given that there is no cure for the condition?
- How can the classifications of cannabis either affect ongoing research and development of approaches for treating various forms of epilepsy?
Incidence
Epilepsy (idiopathic and secondary) is one of the most common chronic, non-noncommunicable neurological disorders worldwide; it is characterized by seizures and affects more than 50 million people (5). The annual incidence rate of epilepsy (two or more unprovoked seizures) is greater than 60 per 100,000 person-years with a higher incidence in low/middle-income countries (LMIC) than in high-income countries (6).
Low- and middle-income countries account for 80% of individuals with epilepsy and the risk of premature death in people with epilepsy is up to three times higher than for the general population (6). There are one hundred and fifty thousand new cases of epilepsy diagnosed in the United State each year, with children and older adults being the fastest-growing segments of the population with new cases (20).

Self Quiz
Ask yourself...
- How do socioeconomic factors contribute to the prevalence of epilepsy?
- What implications do these factors have for equitable healthcare delivery and policymaking?
- What unique challenges do children and older adults face in the diagnosis, treatment, and management of epilepsy?
- How should healthcare providers adapt to meet these specific demographic needs?
Pathophysiology
The pathophysiology of epilepsy is complex and not fully understood. Acquired and genetic factors play a role in about two-thirds of cases (12). Epigenetic modifications can alter gene expression without altering the DNA sequence (13). These modifications can influence the excitability of neurons and contribute to seizure development. Prolonged or excessive neuronal excitation can lead to excitotoxicity, causing damage to neurons, which contribute to the chronic nature of epilepsy.
Normal brain function relies on a delicate balance between excitatory and inhibitory neuron signaling of electrical impulses, or action potentials. In epilepsy, there is an increased propensity for neurons to become excitable, leading to the generation of abnormal electrical discharges.
Seizures result from the hypersynchronous discharge of neurons (7). Anomalies in ion channels (sodium, potassium, calcium) across neuronal cell membrane via mutations in genes encoding that can disrupt normal function, leading to hyperexcitability and increased susceptibility to seizures (7) (10).
Synaptic transmission dysfunction (glutamate and inhibitory GABA neurotransmitters) that can disrupt the normal synaptic transmission, or neural networks that contribute to instability (14). Neuro-anatomical abnormalities (epileptic foci) that can create areas of focal hyperexcitability including tumors, cortical dysplasia, or scar tissue from previous brain injuries (15).
Neuroinflammation and immune system dysregulation can lower the seizure threshold via inflammatory molecules that have pathogenic roles in epilepsy (16).

Self Quiz
Ask yourself...
- What are ways that our current gaps in understanding limit the development of more targeted and effective treatments for the various forms of epilepsy?
- What potential does epigenetic research hold for future therapeutic approaches to epilepsy?
- Can you think of ethical considerations that may arise from interventions?
- How should pharmacological treatments evolve to address these specific biological mechanisms involving neurotransmission more effectively?
- How can advancements in research on the immune system’s role and seizure threshold in epilepsy contribute to the development of therapeutic strategies?
Signs and Symptoms
The hallmark of epilepsy is seizures. Seizures can take various forms including temporary confusion, momentary lapses of attention, severe unprovoked seizures, uncontrollable jerking movements of the limbs, and complete loss of consciousness, bowel, and bladder control (12). There can be changes in sensation including tingling, numbness, or pain in a specific part of the body.
Changes in behavior including staring, confusion, or automatic movements, such as lip smacking or chewing, and changes in vision including flashing lights, blurry vision, or changes in color perception. There can also be changes in hearing including ringing in the ears, buzzing, or auditory hallucinations (5).
Stress, sleep deprivation, and certain medications can trigger an epileptic seizure. Some individuals with epilepsy experience a warning sign or aura (single foci seizure) which manifests as strange sensations, unusual emotions, or even hallucinations (11).
After a seizure, individuals often experience a postictal state of drowsiness, hypertension, headache, nausea, confusion, disorientation, and memory problems which can last for minutes to hours (17). Seizure classification depends on onset location, an individual’s awareness, and whether the seizures involve other symptoms, such as movement.
Absence seizures (petit mal seizures) can involve a brief loss of consciousness, staring, or isolated muscle twitches lasting only a few seconds (18).
Tonic seizures can involve a stiffening of the body, loss of consciousness, and the loss of bowel and or bladder control (18). Clonic seizures can involve jerking movements of the arms and legs and the loss of consciousness (18). Sudden, brief muscle jerks can signify myoclonic seizures (18). Tonic-clonic seizures (grand mal seizures are the most common type of generalized seizure characterized by a stiffening of the body, followed by jerking movements of the arms and legs. Individuals may lose consciousness and control of their bladder or bowel.

Self Quiz
Ask yourself...
- How does the location of seizure onset and an individual’s level of awareness influence the classification of the seizure?
- Given that stress, sleep deprivation, and certain medications can function as triggers for epileptic seizures, what preventative measures can help minimize these triggers?
- How do the auras or warning signs experienced by some individuals prior to a seizure affect their ability to prepare for or manage an impending seizure?
- Considering the range of postictal symptoms like drowsiness, hypertension, and memory issues, how can healthcare professionals better assist individuals in the recovery phase following a seizure?
Causes
The ILAE Commission (1989) classifies focal epilepsies according to their topographical and anatomical origin, including Temporal lobe epilepsies, Frontal lobe epilepsies, Parietal lobe epilepsies and Occipital lobe epilepsies (19).
The various etiologies that categorize epilepsy include idiopathic (unknown cause), symptomatic (known cause), or cryptogenic (presumed symptomatic but cause not identified) (19).
Up to 10% of individuals worldwide have one seizure during their lifetime, which does not signify epilepsy (7). Epilepsy is characterized by recurrent seizures, brief episodes of involuntary movement involving a part of the body (partial) or the entire body (generalized), and sometimes the loss of consciousness and control of bowel or bladder function.
Physiologic factors that can contribute to the onset of epilepsy include genetic predisposition, traumatic brain injury, infections affecting the central nervous system (e.g., meningitis), brain tumors or strokes, developmental disorders, such as autism, and metabolic or biochemical imbalances (11).
Epileptogenicity is the tendency to develop tissue that can cause epileptic seizures via abnormal cellular excitability arising from depolarization and hyperpolarization events (9). Epileptogenesis changes in brain tissue can generate sustained, recurrent seizures following an epileptogenic insult or injury resulting in the progression of epilepsy (8). These changes within the brain involve two stages following the initial insult including the latent period and the chronic epilepsy phase. Epileptogenesis can lead to a permanent state of hyperexcitability, altered connectivity in the brain and future seizure activity (9).

Self Quiz
Ask yourself...
- How does classifying focal epilepsies according to their origin in specific brain lobes guide the treatment approach and prognosis?
- Given the categories of idiopathic, symptomatic, and cryptogenic epilepsy, how might the understanding or lack of understanding of the root cause affect treatment strategies and patient outcomes?
- What are ways that early identification and management mitigate the progression of epileptogenesis?
- Given that epileptogenic changes can lead to a permanent state of hyperexcitability and altered brain connectivity, what are the potential avenues for halting or reversing these changes?
Common Treatments for Epilepsy and Seizures
Antiseizure Medications
Antiseizure medications (ASM) are the most common and primary treatment for epilepsy, although most ASMs do not suppress all types of seizures (21). These medications most often work by several mechanisms, including the modulation of voltage-gated ion channels, by the enhancement of gamma aminobutyric acid-mediated inhibition, through interactions with elements of the synaptic release machinery, by blockade of ionotropic glutamate receptors, or by a combination of these mechanisms (21).
The goal of epilepsy therapy is the complete elimination of seizures, which is not achievable in about one-third of patients (21). There are over 30 different ASMs used in the treatment of epilepsy, including first generation, second generation and third generation drugs (Fig. 1).
Patients with epilepsy require individualized treatment protocols based on the epilepsy syndrome, seizure type, adverse effects profile, pharmacokinetic profile, potential drug interactions, comorbidities age of the patient, reproductive considerations, and cost (21).
Figure 1.
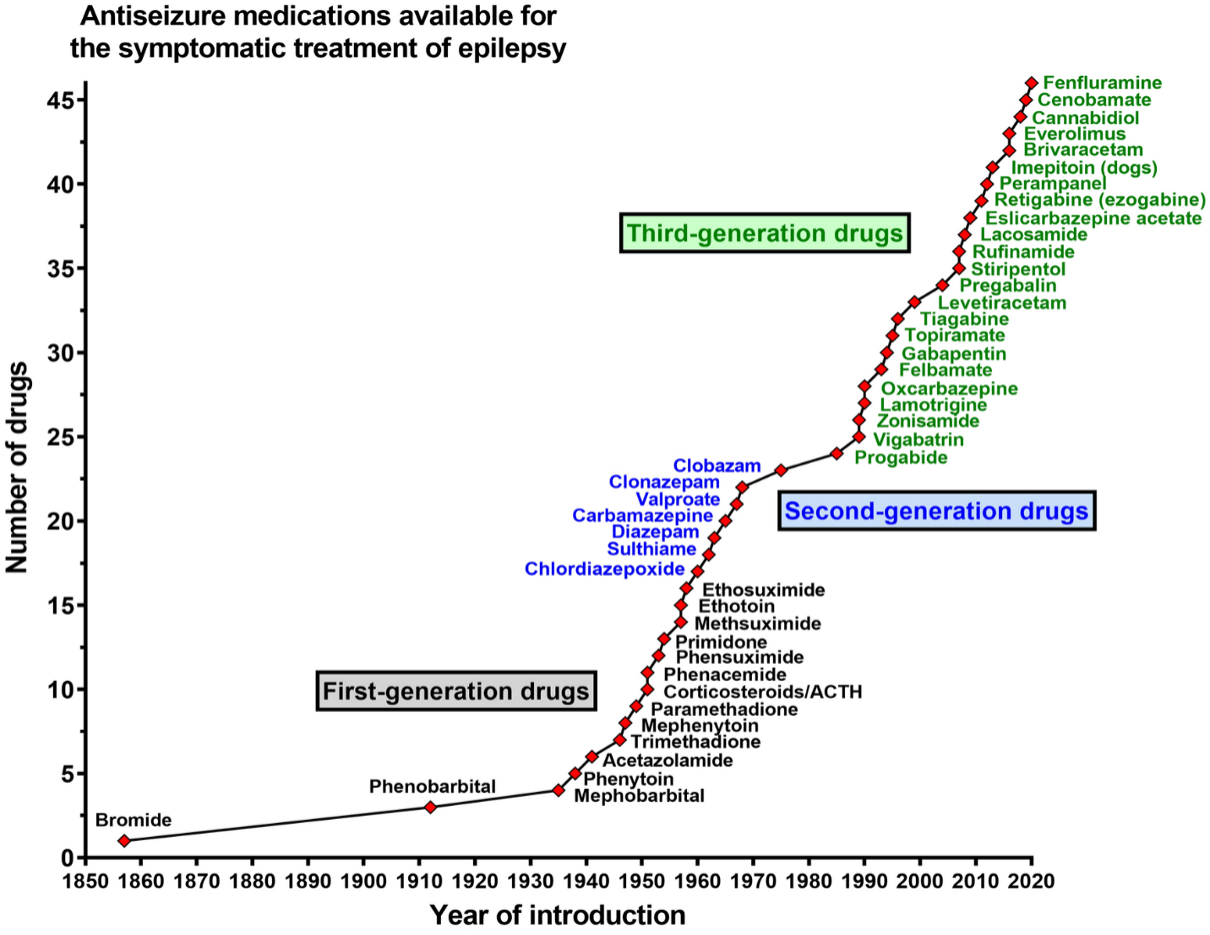
(21)
The 10 most common antiseizure medications for focal onset and generalized seizures include: Lamotrigine (Lamictal), Gabapentin (Neurontin), Levetiracetam (Keppra, Spritam), Phenytoin (Dilantin), Zonisamide (Zonegran); these are used to treat focal onset seizures in combination with other seizure medications: Carbamazepine (Tegretol), Oxcarbazepine (Trileptal), Valproic acid derivatives (Valproic acid and Divalproex), Topiramate (Topamax) and Phenobarbital (22).

Self Quiz
Ask yourself...
- How do the varied mechanisms of action among ASMs influence the selection of medication for distinct types of epilepsy and individual patients?
- If the complete elimination of seizures is unattainable for almost one-third of epilepsy patients despite medication, how should clinicians reconcile this reality with the primary goals of epilepsy treatment?
- What criteria should healthcare providers use to navigate first, second, and third generation antiseizure medications?
- How do advancements in drug development affect treatment choices?
Alternative Approaches
When antiseizure medications (ASMs) are ineffective or when individuals seek complementary or alternative approaches to manage their epilepsy, non-conventional treatments are typically considered.
Some common dietary treatments for epilepsy include the Ketogenic Diet (KD), a high-fat, low-carbohydrate diet shown to reduce seizures in some individuals with epilepsy through the alteration of brain metabolism (23). The Modified Atkins Diet (MAD) is designed to help control seizures in cases where the ketogenic diet may not be suitable (24).
Vagus Nerve Stimulation (VNS) is a surgical procedure where an implanted device connected to the Vagus nerve delivers mild, regular electrical impulses, helping to reduce the frequency and severity of seizures (25).
Responsive Neurostimulation (RNS) is another surgical option for drug-resistant epilepsy, which involves implanting a neurostimulator device in the brain, which monitors brain activity; if there is detection of abnormal activity, it delivers electrical stimulation, aiming to prevent seizures (26).
For certain cases of epilepsy, surgical resection, involving removal of the part of the brain responsible for seizures, or disconnecting it from the rest of the brain. The specific procedure depends on the location of the seizures, but can include a lesionectomy to remove lesions, lobectomy to remove a lobe (part of the brain responsible for seizures), multi-lobar resection to remove two or more lobes of your brain, or hemispherectomy which removes or disconnects one-half of the brain (27).
Additional surgical options include disconnection or cutting the communication between the area of the brain generating the seizures. Surgical disconnection can include a Corpus callosotomy which involves cutting the corpus callosum (the main fiber bundle connecting the hemispheres of the brain) and multiple subpial transections which involves making several shallow cuts into a limited section of brain tissue which stop the communication between nerve cells where seizures are happening and other normal nerve cells (27).
Transcranial Magnetic Stimulation (TMS) is a non-invasive procedure that uses magnetic fields to stimulate specific areas of the brain which has shown potential as a treatment for epilepsy in research studies (28).
In addition to the specific treatments mentioned above, there are several lifestyle modifications including adequate sleep, managing stress, avoiding alcohol and recreational drugs, and maintaining a regular schedule can be essential in minimizing seizure triggers (29).
The use of vitamin B6, magnesium, or omega-3 fatty acids as adjunctive treatments and alternative therapies such as acupuncture or biofeedback to help manage stress can be essential in minimizing seizure triggers (30). The effectiveness of these non-medication treatments can vary from person to person, and not all individuals will respond to these approaches.

Self Quiz
Ask yourself...
- How can standardized treatment guidelines be both comprehensive and adaptable?
- How should these non-medication treatments be integrated into a broader treatment plan, and what ethical considerations come into play when recommending surgical procedures like Vagus Nerve Stimulation or brain resection?
- What factors should clinicians consider when recommending dietary changes as a treatment strategy?
- Can you discuss the use of therapies such as vitamin supplements and acupuncture?
History and Background on Medical Cannabis Use
The 20th century saw the global scrutiny of cannabis due to concerns about its psychoactive effects and potential for abuse (32). These psychoactive effects have limited the use of Cannabis in clinical practice and led to stigmatization, making it difficult for researchers to study the potential medical benefits. According to the World Health Organization (WHO), Cannabis is the most cultivated, trafficked, and abused illicit drug worldwide with an annual prevalence rate of 147 million individuals or 2.5% of the global population (33).
However, throughout history, medical practitioners have used cannabis to treat a wide range of ailments, including pain, inflammation, and neurological conditions. Evidence has suggested it was used more than 5,000 years ago in modern-day Romania (32). The achenes (seeds) of cannabis have documented use in Chinese medicine for at least 1800 years (32). The industrial cultivation of Cannabis (marijuana) for its fiber, seeds, medicinal and psychoactive properties has occurred for thousands of years. The first recorded use of cannabis for epilepsy was in ancient Egypt, around 1500 BC (31).
In recent years, there has been a renewed interest in the use of cannabis for medical purposes, including epilepsy, due in part to the growing body of research suggesting its effectiveness. In the late 20th century, scientific research revealed the existence of the endocannabinoid system (ECS) in the human body and the biological effects of cannabinoids on the central nervous system.
The major constituents of Cannabis sativa (marijuana) and two primary cannabinoid receptors (CB1R) and (CB2R) are the prominent subtypes with potential therapeutic avenues in several pathological conditions, including neuropsychological disorders, neurodegenerative diseases, pain perception, and neuronal excitability (34).

Self Quiz
Ask yourself...
- Considering the long historical use of cannabis for medical purposes and its recent resurgence in scientific research, how do you think healthcare systems should reconcile the drug’s current Schedule I status with emerging evidence of its therapeutic benefits, particularly in the treatment of epilepsy?
- Given the discovery of the endocannabinoid system and its role in various pathological conditions, how might this biological understanding influence the development of cannabinoid-based medications and what are the regulatory challenges posed by such advancements?
- How can healthcare professionals balance the potential for abuse against the possible medical benefits, especially in the case of neurological conditions like epilepsy?
- Can you think of ethical and cultural considerations that practitioners should address in the current debate surrounding cannabis use for medical purposes, including epilepsy?
State and Federal Regulations
As of April 2023, medical cannabis is legal in 38 states, three out of five inhabited U.S. territories, and the District of Columbia (35). Individual states have their own medical cannabis laws in terms on qualifying conditions, the purchasing of medical cannabis, and the regulation of the medical cannabis industry.
Most U.S. states have enacted medical cannabis laws, allowing patients with epilepsy and other qualifying conditions to access cannabis-based treatments. To obtain medical cannabis, patients must first obtain a recommendation from a licensed healthcare provider.
Medical cannabis is available in a variety of forms, including dried flower, edibles, vaporized concentrates, and topical products (36).
Cannabis remains a Schedule I substance under federal law alongside heroin, peyote, ecstasy, and LSD, and considered to have a high potential for abuse, without any accepted medical use. The Schedule I designation has made it difficult for scientists to study. As a result, it is a federal crime to use, buy, sell, or possess cannabis (35).
On December 2, 2022, President Biden enacted the Medical Marijuana and Cannabidiol Research Expansion Act (HR 8454), thereby streamlining the process for conducting cannabis-related medical research within the United States. HR 8454 is the first stand-alone marijuana-related bill approved by both chambers of the United States Congress. The Epilepsy Foundation has been a fervent advocate for this legislative measure and anticipates its forthcoming execution (36). For individuals with epilepsy, the Medical Marijuana and Cannabidiol Research Expansion Act enacted law permitting physicians to engage in candid discussions with their patients about the potential advantages and drawbacks of using cannabis as a medical treatment, without contravening the Controlled Substances Act.
This clarification alleviates any previous hesitations among healthcare providers, who often refrained from discussing cannabis and CBD usage due to concerns about legal implications. This legislative amendment will enhance the dialogue between epileptic patients and their healthcare professionals, thereby facilitating more effective treatment strategies and outcomes.


Self Quiz
Ask yourself...
- Given that medical cannabis is legal in 38 states but remains a Schedule I substance under federal law, how should healthcare providers navigate this discrepancy when discussing cannabis-based treatments with their patients with epilepsy?
- What barriers still exist for scientists who wish to conduct cannabis-related medical research, and how might these barriers impact the development of evidence-based treatments for conditions like epilepsy?
- How can the Epilepsy Foundation’s strong advocacy for the Medical Marijuana and Cannabidiol Research Expansion Act influence future healthcare policy and clinical guidelines?
- What steps should healthcare systems take to ensure that both providers and patients stay informed about the latest research and treatment options?
Indication of Use for Epilepsy
Epilepsy and Cannabidiol (CBD) is one of the most well-studied cannabinoids in cannabis. However, it is important to weigh the risks and benefits of medical cannabis. Medical cannabis is not a cure for epilepsy or a replacement for antiseizures medications (ASM), but a treatment option that can help to reduce seizures and improve quality of life.
Medical cannabis can interact with other medications, including antiepileptic drugs. While the Cannabis sativa plant contains an estimated 100 cannabinoids, the primary compounds with potential antiepileptic properties include Δ9-tetrahydrocannabinol (THC), Δ9-tetrahydrocannabivarin, cannabidivarin, Δ8-tetrahydrocannabinol, cannabinol, and cannabidiol (CBD). Various pharmaceutical and non-pharmaceutical products on the market incorporate CBD and/or THC (41).
In 2013, one of the earliest landmark cases that drew attention to the potential of medical cannabis in epilepsy was that of Charlotte Figi, a young girl with Dravet syndrome, a severe form of epilepsy attributed to a spontaneous genetic mutation. Her story, which became known nationwide as the “Charlotte’s Web” case, involved the use of a high-CBD cannabis extract that reduced her seizures, which had reached 350 seizures per week (38). After a battle with pneumonia, an illness that triggered a resurgence of her seizures, Charlotte passed away on April 8, 2020 (38).
Research continues to focus on the potential of cannabis as an antiseizure medication. Multiple preclinical and clinical studies demonstrated promising results, suggesting that CBD could reduce the frequency and severity of seizures in various forms of epilepsy.
A 2018 study found that cannabidiol (CBD), one of the main compounds in cannabis, was effective in reducing seizures in children with Dravet syndrome, a rare form of epilepsy (39). In 2018, the U.S. Food and Drug Administration granted approval for CBD for use as an adjunctive antiepileptic medication for children as young as two years old with diagnosed Dravet syndrome and Lennox-Gastaut syndrome (40). Lennox-Gastaut syndrome and Dravet syndrome are childhood-onset epilepsies and are among the most difficult to treat (41).
This marked the first FDA-approved medication derived from cannabis. The European Medicines Agency extended its approval for the drug in 2019. The pharmaceutical-grade, purified plant-derived CBD preparation is available under the names Epidiolex or Epidiolex, distributed by GW Pharmaceuticals (40).
While the approval of Epidiolex was a breakthrough, several challenges and concerns remained. These included issues related to the regulation and standardization of medical cannabis products, the need for further research on optimal dosing and long-term effects, and the variation in state laws regarding the use of medical cannabis.
Research into medical cannabis and epilepsy continued to expand. Clinical trials and observational studies aimed to further understand the effectiveness and safety of various cannabis-based therapies for several types of seizures and epilepsy syndromes.
One of the ongoing challenges in the use of medical cannabis for epilepsy is determining the most effective dosages and formulations. Variability in cannabis strains, extraction methods, and product consistency can make it difficult for patients and healthcare providers to find the most suitable treatment (44). Ongoing research, regulatory developments, and patient advocacy will continue to shape the role of medical cannabis in the treatment of epilepsy and offer hope to individuals seeking alternatives to traditional antiseizure medications.
There is limited evidence for the effectiveness of tetrahydrocannabinol (THC) and other cannabinoids in treating epilepsy in humans. As of 2018, there is now class 1 evidence that adjunctive use of CBD improves seizure control in patients with specific epilepsy syndromes. The completion of three high-quality placebo-controlled adjunctive-therapy trials of a purified CBD product in patients with Dravet syndrome and Lennox-Gastaut syndrome found the CBD product to be superior to placebo in reducing the frequency of convulsive (tonic-clonic, tonic, clonic, and atonic) seizures among children and adults with the Lennox–Gastaut syndrome.
The addition of cannabidiol at a dose of 10 mg or 20 mg per kilogram per day to a conventional antiepileptic regimen resulted in greater reductions in the frequency of drop seizures than placebo (43). It is unclear if the enhanced seizure management observed in these trials was a direct result of CBD’s effects or if the result of the interactions with other antiseizure medications influenced the results.
- Cannabidiol (CBD)
Class I Evidence: Epidiolex, a pharmaceutical-grade CBD, has received FDA approval for the treatment of certain forms of epilepsy, namely Dravet syndrome and Lennox-Gastaut syndrome, in patients 2 years of age and older. Multiple randomized controlled trials provide Class I evidence for the efficacy of CBD in these conditions.
- Tetrahydrocannabinol (THC):
Class I, II, III Evidence: High-quality evidence supporting the efficacy of THC in treating epilepsy is not abundant. THC has psychoactive properties and can cause cognitive impairment, which makes it less ideal for treating conditions like epilepsy. Most clinical guidelines do not recommend THC as a treatment option for epilepsy due to the lack of robust clinical evidence (45).
- Other Cannabinoids:
Class I, II, III Evidence: The evidence for other cannabinoids like cannabidivarin (CBDV) and cannabinol (CBN) is even more limited and does not reach Class I or even Class II standards. Most of these studies are in pre-clinical stages, involving animal models (46).
For reference:
Class I evidence is the highest level of evidence and consists of randomized controlled trials (RCTs) or meta-analyses of multiple clinical trials with substantial treatment effects.
Class II evidence includes well-designed cohort or case-control analytic studies.
Class III evidence includes case reports, case series, and expert opinions (47).

Self Quiz
Ask yourself...
- How does the classification of evidence levels (Class I, Class II, Class III) impact the credibility and applicability of cannabis-based treatments in epilepsy management?
- What ethical considerations arise from the case of Charlotte Figi, especially in terms of promoting CBD as a treatment option for severe epilepsy?
- In what ways do state laws regarding the use of medical cannabis influence treatment options and patient outcomes for those with epilepsy?
- Can you discuss any challenges that remain for healthcare providers in determining appropriate dosages and formulations?
Clinical Criteria
It is important to recognize that medical cannabis is not appropriate for all individuals with epilepsy and requires supervision by healthcare professionals. The consideration around medical cannabis is often for individuals who have intractable seizures that have not responded to traditional antiseizure medications.
Key principles in the medical management of drug-resistant epilepsy:
Accurate Diagnosis – The cornerstone of epilepsy management lies in its accurate diagnosis, which is dependent on a comprehensive clinical assessment. This should be corroborated by diagnostic tools such as EEG, video-EEG, MRI scans, home-based video footage, and genetic testing. These modalities are instrumental in identifying the type of epilepsy syndrome, as well as ruling out conditions that may mimic epileptic seizures (42).
Targeted Antiseizure Medication (ASM) Monotherapy – The gold standard for treatment is to initiate antiseizure monotherapy via a guided diagnosis of epilepsy syndrome dictated by the specific seizure type (42).
Alternate Strategies for Drug-Resistant Epilepsy (DRE) patients – For those patients who exhibit drug resistance, evidenced by the failure of at least two suitable ASMs, a shift toward alternative therapeutic options requires consideration. The management of drug-resistant epilepsy is a complex, evolving field that requires a multifaceted approach and the expertise of specialized healthcare providers. As such, adherence to these guiding principles is crucial for optimizing patient outcomes (42).

Self Quiz
Ask yourself...
- How might the interactions between CBD and existing antiseizure medications (ASMs) affect the overall efficacy of epilepsy treatment? Is it more beneficial or risky?
- What role do diagnostic tools like EEG, video-EEG, and MRI scans play in determining if medical cannabis could be an effective treatment for a specific individual with epilepsy?
- In the context of drug-resistant epilepsy (DRE), how should medical professionals weigh the risks and benefits of alternative treatments like medical cannabis against traditional antiseizure medications?
- What do you think future research should prioritize to address the limitations and inconsistencies in current studies on the effectiveness of cannabinoids in treating epilepsy?
Mechanism of Action
The exact mechanism of action of CBD on epilepsy is a complex pharmacological profile and involves various physiological systems to produce its antiepileptic effects. Cannabidiol (CBD), a non-intoxicating constituent of cannabis, has decreased seizure activity in several types of childhood epilepsy.
Researchers from NYU Grossman School of Medicine conducted a study that confirmed CBD inhibits the signals transmitted by a molecule known as lysophosphatidylinositol (LPI) (48). Lysophosphatidylinositol, located in neuronal brain cells, can intensify nerve impulses under regular conditions. However, in disease states, Lysophosphatidylinositol (LPI) may facilitate seizure activity (48). Other possible therapeutic targets of CBD encompass ion channels, transporters, and transmembrane signaling protein (49).
Anti-inflammatory Actions
Cannabidiol (CBD) has anti-inflammatory properties and neuroprotective activity, which may help to reduce the risk of seizures (51) (55). Clinical studies have confirmed that CBD may reduce the release of pro-inflammatory cytokines and cytokine modulation; thereby reducing neuroinflammation, which can play a role in seizure activity (56).
Studies have indicated elevated cytokines levels in epilepsy patients and moreover, provide direct clinical evidence that the immune system and cytokines mediators play a role in epilepsy disorders (57).
GABA (γ-Aminobutyric acid)
Cannabidiol (CBD) can increase the levels of the inhibitory neurotransmitter GABA, a neurotransmitter that helps to relax the nervous system, which can help to reduce seizures.
Clinical study evidence indicates that GABAergic modulation (the principal inhibitory neurotransmitter in the cerebral cortex) has a role in the mechanism and treatment of epilepsy (50).
Ion Channels
CBD exhibits a strong affinity for ion channels, particularly the Transient Receptor Potential Vanilloid (TRPV), and TRPV1. This non-selective channel is permeable to calcium ions (Ca2+) and plays a significant role in both seizure modulation and epilepsy management (51).
Glutamine
Glutamine is the precursor to glutamate, which serves as the primary excitatory neurotransmitter in the adult mammalian brain. Research on epilepsy has historically focused on understanding its role in seizure activity. Seizures trigger an increase in extracellular glutamate, leading to excitotoxic harm (52).
Long-term seizure activity can modify the expression of glutamate receptors and uptake transporters in both neurons and glial cells, exacerbating the development of epilepsy. There is evidence suggesting that glutamate dysfunction is a common factor in epilepsy as well as other central nervous system (CNS) conditions, such as depression, which frequently co-occurs with epilepsy (52).
CBD can reduce the release of excitatory neurotransmitters, which may help to reduce the risk of seizures (52). Preliminary studies in the preclinical stage indicate that cannabidiol (CBD) exerts its multi-faceted pharmacological effects by regulating levels of excitatory glutamate and inhibitory γ-aminobutyric acid (GABA) neurotransmitters in the brain (53).
Endocannabinoid system (ECS)
CBD interacts with the endocannabinoid system, which is a network of receptors and signaling molecules that plays a role in a variety of bodily functions, including mood, pain, and appetite. Research indicates that the endocannabinoid system participates in the development of epilepsy, and CBD may work by modulating the endocannabinoid system to reduce seizures (54). The research demonstrates how the ECS can contribute to neuroinflammation and therefore modulated by cannabinoids to reduce the incidence and severity of seizures (54).
Inhibition of Adenosine Reuptake
CBD inhibits the reuptake of adenosine, which increases its concentration in the brain and may result in anti-inflammatory and neuroprotective effects. Furthermore, cannabidiol (CBD) influences adenosine levels by inhibiting its reuptake. This action has been correlated with neuroprotective and anti-inflammatory mechanisms within the brain (59).
5-HT1A Receptor
CBD has affinity for the 5-HT1A serotonin receptor, potentially mood-stabilizing and anti-anxiety effects, although the relevance of this to its antiepileptic effects. Evidence indicates that cannabidiol (CBD) induces anxiolytic and antiepileptic effects through the activation of 5-HT1A receptors (59).

Self Quiz
Ask yourself...
- What is the biological significance of CBD’s interaction with lysophosphatidylinositol (LPI) in neuronal brain cells?
- Given that CBD has anti-inflammatory properties and can modulate cytokine release, how could these actions contribute to its efficacy in treating epilepsy compared to its other pharmacological profiles?
- How do the actions of CBD on GABAergic modulation differ from traditional antiseizure medications?
- Considering CBD’s affinity for ion channels, specifically TRPV and TRPV1, how might this feature differentiate CBD from other antiepileptic drugs in terms of safety and efficacy?
Contraindications
Cannabidiol (CBD) has garnered attention for its potential therapeutic benefits in treating various conditions, including epilepsy. However, it is crucial to be aware of contraindications and potential interactions when using CBD alongside other treatments for epilepsy.
Drug Interactions
CBD may interact with antiseizure drugs (AEDs) such as clobazam, valproate, and others. It has the potential to affect their metabolism and efficacy. The liver metabolizes both CBD and several antiseizure medications (61). Individuals with other comorbidities such as liver or kidney diseases may have complications when using CBD, as these organs are often involved in drug metabolism. Concurrent use could result in liver enzyme abnormalities, requiring close monitoring.
There is still limited knowledge regarding the ideal dosing of CBD for epilepsy, in combination with other ASMs (62). Incorrect dosing could either potentiate or negate the effects of other medications. CBD may amplify the sedative effects of certain ASMs, leading to heightened drowsiness or cognitive impairment (60).
The safety of CBD during pregnancy is not well-studied, and practitioners should advise patients to avoid CBD if they are pregnant or planning to become pregnant, given that some ASMs exist with pregnancy-related risks (63).
The purity and concentration of over-the-counter CBD products can vary, making it difficult to ascertain the specific effects or potential interactions with ASMs (64). While CBD is legal in many jurisdictions, its legal status varies by country and state, which could impact its use as a concurrent treatment option. Practitioners should consider the long-term impact of CBD usage, in conjunction with AMSs, as the topic remains understudied.
Cannabidiol–Psychoactive Drug Interactions:
Carbamazepine (Anti-epileptic drug) – Will decrease the level or effect of cannabidiol by affecting hepatic/intestinal enzyme CYP3A4 metabolism (60).
Lamotrigine (Anti-epileptic drug) – CBD will increase the level or effect of lamotrigine by inhibiting UGT2B7 activity (60).
Oxcarbazepine (Anti-epileptic drug) – Will decrease the level or effect of CBD by affecting hepatic/intestinal enzyme CYP3A4 metabolism (60).
Phenobarbital (Anti-epileptic drug) – Will decrease the level or effect of CBD by affecting hepatic/intestinal enzyme CYP3A4 metabolism or CYP2C19 metabolism CBD potentiates the anticonvulsant effect of phenobarbital (60).
Phenytoin (Anti-epileptic drug) – Will decrease the level or effect of CBD by affecting hepatic/intestinal enzyme CYP3A4/CYP2C19 metabolism. CBD may potentiate the anticonvulsant effects of phenytoin (60).
Chlordiazepoxide, Clonazepam, Ethosuximide – CBD reduces the anticonvulsant effects of these drug (60).
Clobazam or Diazepam (Benzodiazepine) – CBD will increase the level or effect of clobazam or diazepam by affecting hepatic enzyme CYP2C19 metabolism. CBD increases clobazam plasma concentrations (60).
Morphine (Opioid) – CBD will increase the level or effect of morphine (60).
Desipramine (Tricyclic anti-depressant) – Desipramine will increase the level or effect of CBD by affecting hepatic/intestinal enzyme CYP3A4 metabolism (60).
Imipramine (Tricyclic anti-depressant) – CBD will increase the level or effect of imipramine by affecting hepatic enzyme CYP2C19 metabolism (60).
Trimipramine (Tricyclic anti-depressant) – CBD will increase the level or effect of trimipramine by affecting hepatic enzyme CYP2C19 metabolism (60).

Self Quiz
Ask yourself...
- Could the regulation of excitatory neurotransmitters like glutamate by CBD be a universal mechanism for the treatment of other central nervous system disorders, such as depression, that often co-occur with epilepsy?
- How might the interaction of CBD with the endocannabinoid system present opportunities or challenges in the development of targeted therapies for epilepsy?
- In the context of drug interactions, how might healthcare providers mitigate the risks associated with using CBD in conjunction with traditional antiseizure medications?
- In the context of drug interactions, how might healthcare providers mitigate the risks associated with using CBD in conjunction with traditional antiseizure medications?
Common Side Effects
CBD is not risk-free. While considered safe and well tolerated, it is important to note that CBD may cause some side effects when taken in higher doses or when taken with certain medications.
The most common side effects of CBD include:
- Drowsiness or fatigue
- Dry mouth
- Diarrhea
- Nausea
- Liver Toxicity
- High doses may have potential liver toxicity, although this is rare (61).
- Mood Changes
- While CBD can treat anxiety and depression, some individuals might experience mood swings or irritability.
- Changes in Appetite
- CBD might either suppress or stimulate appetite, depending on the individual and the dosage (65).
- Light-headedness
- Lowered blood pressure can lead to feelings of light-headedness in some users (66).
- Interactions with Other Medications
- CBD could interact with prescription medications, including blood thinners.

Self Quiz
Ask yourself...
- How should healthcare providers weigh the risks and benefits when recommending CBD, especially in patients with pre-existing conditions?
- How might the interaction of CBD with other medications, such as blood thinners, impact its safety profile?
- Can you name precautions for patients who are already on a multi-drug regimen?
Nursing Implications
Nurses play a significant role in educating and supporting patients who are using CBD. The primary considerations include an awareness of drug interactions and side effects. Nurses should assess the patient’s medication list to identify any potential interactions and educate the patient about the risks and benefits.

The most common side effects of CBD are mild and go away on their own. However, nurses should be aware of the potential for more serious side effects, such as drowsiness, fatigue, dry mouth, diarrhea, reduced appetite, nausea, and liver damage. Nurses should monitor patients for side effects and educate them about how to manage them.
There is no standard dose for CBD, and the optimal dose may vary depending on the patient’s condition, other medications they are taking, and their individual response (72) (74). Nurses can help patients to determine a safe and effective dose by reviewing their medical history and monitoring their response to CBD.
Route of administration for CBD vary, including oils, capsules, edibles, and topicals (73). The route of administration can affect the absorption and onset of action of CBD. Nurses should educate patients about the different routes of administration and help them to choose the one that is best for them.
CBD may cause drowsiness and fatigue, which could impair judgment and coordination (70). Nurses should educate patients about this potential risk and advise them to avoid driving or operating machinery while using CBD.
Nurses should inform pregnant or breastfeeding women to avoid CBD. Nurses should educate patients about this risk and advise them to talk to their doctor before using CBD if they are pregnant or breastfeeding (71).
The legal status of CBD varies from country to country and state to state. Nurses should be aware of the legal status of CBD in their jurisdiction and advise patients in accordance with local and state laws.


Self Quiz
Ask yourself...
- How can nurses develop a robust methodology for helping patients determine the most effective and safest dose, especially when patients are already taking other medications?
- How should nurses equip themselves to provide accurate and ethical guidance to patients about the use, possession, and procurement of CBD?
- How can nurses ensure they are knowledgeable to recommend the most appropriate method for individual patients?
- Can you discuss ways that nurses can monitor for side effects and educate patients on their management?
Current and Upcoming Research
The therapeutic potential of Cannabidiol (CBD) in the treatment of epilepsy has garnered substantial attention from the scientific community and healthcare professionals alike. The medical profession requires long-term studies to assess the safety and effectiveness of CBD, including any potential for liver toxicity or other adverse effects moving forward (33).
Epidiolex is the only FDA-approved CBD-based medication for treating specific types of epilepsy (Lennox-Gastaut syndrome and Dravet syndrome) in patients two years of age and older. Essentially, the options are very limited. There is a growing interest in studying the safety and efficacy of CBD in children with epilepsy types other than Lennox-Gastaut and Dravet syndrome. The research on CBD in other medical conditions is ongoing to understand its long-term effects and applications for other types of epilepsy.
Cannabidivarin (CBDV) is another non-psychoactive cannabinoid undergoing research for its anti-epileptic properties. Initial results indicate that CBDV is an effective anticonvulsant in a broad range of seizure models (67). Several studies are focusing on CBDs interaction with the endocannabinoid system and other neurotransmitter systems, including CB1 and CB2 receptors.
(68)
Current research is evaluating how CBD interacts with traditional antiepileptic drugs, and the consideration that the metabolism of CBD occurs via the cytochrome P450 enzyme system (69).

Self Quiz
Ask yourself...
- What ethical considerations should guide the medical community in recommending or prescribing non-FDA approved CBD products for patients, especially children, with other forms of epilepsy?
- How do you think the scientific community should prioritize the study of various cannabinoids?
Patient Education
Patient education is essential for the safe and effective use of CBD (71). Patients may require the following information regarding the potential benefits and risks. CBD is well-tolerated, but it can cause dry mouth, fatigue, and diarrhea and can interact with other medications, so patients should talk to their doctor before using CBD.
Patients should choose the form of CBD that is most convenient and effective for them. The forms include oils, capsules, edibles, and topical products (73). The appropriate dosage of CBD will vary depending on the patients’ individual needs and the underlying condition. Patients should start with a low dose and increase as needed to achieve the desired effect. Encourage patients to talk to their doctor before using CBD (74). This is important for patients who are taking other medications or who have any underlying health conditions.
By providing patients with accurate and comprehensive information about CBD, nurses can help them use this natural therapy safely and effectively (71).

Self Quiz
Ask yourself...
- How would you describe the role of healthcare providers, particularly nurses, for a more structured approach to CBD education?
- In the context of patient education, what challenges could arise from the lack of standardized dosing guidelines for CBD, and how can healthcare providers mitigate these challenges to ensure patient safety and efficacy of treatment?
Conclusion
The potential of Cannabidiol (CBD) as a therapeutic agent in the treatment of epilepsy represents a significant advancement in neurology and pharmacology. With FDA approval for Epidiolex, targeting Lennox-Gastaut and Dravet syndromes, CBD has transitioned from experimental status to a legitimate treatment option for certain types of epilepsy. The effectiveness of CBD in reducing seizure frequency and severity, often where conventional antiepileptic drugs have failed, opens new avenues for the management of this complex neurological disorder.
However, the integration of CBD into the treatment regimen of epilepsy is not without challenges. A primary concern revolves around drug interactions. Healthcare providers must exercise due diligence in medication reconciliation and monitoring when introducing CBD into a patient’s treatment plan.
There continues to be an urgent need for more extensive research to understand CBD’s long-term effects, optimal dosing, and efficacy in treating other forms of epilepsy.
Patient education is another crucial area requiring robust strategies, given the excess of information and misinformation available to the public. It is imperative that healthcare providers give patients and caregivers accurate, evidence-based information to help them make informed choices about their treatment options. This includes discussing the potential side effects, legal implications, and cost considerations associated with CBD use.
Given the rapid changes in legislation and the escalating interest in medical cannabis, clinicians must remain responsive, amending to new research findings and incorporating them into clinical practice and patient education protocols.
In summary, while CBD shows promising potential in epilepsy treatment, its integration into mainstream medical practice requires a multifaceted approach, balancing clinical benefits against potential risks, within the framework of ethical, legal, and administrative considerations.

Self Quiz
Ask yourself...
- What strategies could healthcare providers employ to ensure medication reconciliation and monitoring are rigorous when introducing CBD into a patient’s epilepsy treatment plan?
- How can healthcare providers remain agile in amending clinical practice and patient education protocols?
References + Disclaimer
- National Academies Press (US). (2017, January 12). Therapeutic effects of cannabis and cannabinoids. The Health Effects of Cannabis and Cannabinoids – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK425767/
- Patel, P., & Moshé, S. L. (2020). The evolution of the concepts of seizures and epilepsy: What’s in a name? Epilepsia Open, 5(1), 22–35. https://doi.org/10.1002/epi4.12375
- Walshe, T. M. (2016). On the Sacred Disease. In Oxford University Press eBooks (pp. 43–60). https://doi.org/10.1093/med/9780190218560.003.0004
- Cambridge University Press. (2007). An introduction to the life and work of John Hughlings Jackson: Introduction. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2640105/
- Panayiotopoulos, C. P. (2011). The new ILAE report on terminology and concepts for organization of epileptic seizures: A clinician’s critical view and contribution. Epilepsia, 52(12), 2155–2160. https://doi.org/10.1111/j.1528-1167.2011.03288.x
- Beghi, E. (2019). The Epidemiology of Epilepsy. Neuroepidemiology, 54(2), 185–191. https://doi.org/10.1159/000503831
- World Health Organization: WHO. (2023). Epilepsy. www.who.int. https://www.who.int/news-room/fact-sheets/detail/epilepsy
- Rakhade, S. N., & Jensen, F. E. (2009). Epileptogenesis in the immature brain: emerging mechanisms. Nature Reviews Neurology, 5(7), 380–391. https://doi.org/10.1038/nrneurol.2009.80
- Pitkänen, A., Łukasiuk, K., Dudek, F. E., & Staley, K. J. (2015). Epileptogenesis. Cold Spring Harbor Perspectives in Medicine, 5(10), a022822. https://doi.org/10.1101/cshperspect.a022822
- Stafstrom, C. E., & Carmant, L. (2015). Seizures and Epilepsy: An overview for neuroscientists. Cold Spring Harbor Perspectives in Medicine, 5(6), a022426. https://doi.org/10.1101/cshperspect.a022426
- Epilepsy and seizures. (2023). National Institute of Neurological Disorders and Stroke. https://www.ninds.nih.gov/health-information/disorders/epilepsy-and-seizures
- Pal, D. K., Pong, A. W. & Chung, W. K. Genetic evaluation, and counseling for epilepsy. Nature Reviews Neurology 6, 445-453, doi:10.1038/nrneurol.2010.92 (2010).
- Hamilton, J. P. (2011). Epigenetics: Principles and practice. Digestive Diseases, 29(2), 130–135. https://doi.org/10.1159/000323874
- Barker‐Haliski, M., & White, H. S. (2015). Glutamatergic Mechanisms Associated with Seizures and Epilepsy. Cold Spring Harbor Perspectives in Medicine, 5(8), a022863. https://doi.org/10.1101/cshperspect.a022863
- Crino, P. B. (2015). Focal cortical displasia. Seminars in Neurology, 35(03), 201–208. https://doi.org/10.1055/s-0035-1552617
- Vezzani, A., Lang, B., & Aronica, E. (2015). Immunity and inflammation in epilepsy. Cold Spring Harbor Perspectives in Medicine, 6(2), a022699. https://doi.org/10.1101/cshperspect.a022699
- Abood, W. (2023, July 10). Postictal seizure state. Stat Pearls – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK526004/
- Types of seizures | Epilepsy | CDC. (2023). https://www.cdc.gov/epilepsy/about/types-of-seizures.htm
- Scheffer, I. E., Berkovic, S. F., Capovilla, G., Connolly, M., French, J., De Figueiredo Ferreira Guilhoto, L. M., Hirsch, É., Jain, S., Mathern, G. W., Moshé, S. L., Nordli, D. R., Perucca, E., Tomson, T., Wiebe, S., Zhang, Y., & Zuberi, S. M. (2017). ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia, 58(4), 512–521. https://doi.org/10.1111/epi.13709
- Sirven, J. I. (2015). Epilepsy: a spectrum disorder. Cold Spring Harbor Perspectives in Medicine, 5(9), a022848. https://doi.org/10.1101/cshperspect.a022848
- Löscher, W., & Klein, P. (2021). The pharmacology and clinical efficacy of antiseizure medications: from bromide salts to cenobamate and beyond. CNS Drugs, 35(9), 935–963. https://doi.org/10.1007/s40263-021-00827-8
- Le, K. (2022, April 28). The 10 most common antiepileptic seizure medications. GoodRx. https://www.goodrx.com/classes/anti-epileptics/best-antiepileptic-seizure-medication
- Ketogenic diet. (2023). Epilepsy Foundation. https://www.epilepsy.com/treatment/dietary-therapies/ketogenic-diet
- Meira, I. D., Romão, T. T., Prado, H. J. P. D., Krüger, L. T., Pires, M. E. P., & Da Conceição, P. O. (2019). Ketogenic Diet and Epilepsy: What We Know So Far. Frontiers in Neuroscience, 13. https://doi.org/10.3389/fnins.2019.00005
- Vagus Nerve stimulation (VNS) therapy. (2023). Epilepsy Foundation. https://www.epilepsy.com/treatment/devices/vagus-nerve-stimulation-therapy
- Responsive Neurostimulation (RNS). (2023). Epilepsy Foundation. https://www.epilepsy.com/treatment/devices/responsive-neurostimulation
- Epilepsy surgery, Temporal lobectomy, Vagus Nerve Stimulation | Cincinnati Ohio Mayfield Brain & Spine. (2023). https://mayfieldclinic.com/pe-epilepsysurg.htm
- Walton, D., Spencer, D. C., Nevitt, S. J., & Michael, B. (2021). Transcranial magnetic stimulation for the treatment of epilepsy. The Cochrane Library, 2021(4). https://doi.org/10.1002/14651858.cd011025.pub3
- Stress, mood, and seizures. (2023). Epilepsy Foundation. https://www.epilepsy.com/complications-risks/moods-behavior/stress-mood-and-seizures
- Natural approaches to epilepsy. (2007, March 1). PubMed. https://pubmed.ncbi.nlm.nih.gov/17397265/
- Russo, E. B. (2007). History of Cannabis and its preparations in Saga, Science, and Sobriquet. Chemistry & Biodiversity, 4(8), 1614–1648. https://doi.org/10.1002/cbdv.200790144
- Brand, E., & Zhao, Z. (2017). Cannabis in Chinese medicine: Are some traditional indications referenced in ancient literature related to cannabinoids? Frontiers in Pharmacology, 8. https://doi.org/10.3389/fphar.2017.00108
- Bridgeman, M. B. (2017, March 1). Medicinal Cannabis: History, Pharmacology, And Implications for the Acute Care Setting. PubMed Central (PMC). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5312634/
- Zou, S., & Kumar, U. (2018). Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system. International Journal of Molecular Sciences, 19(3), 833. https://doi.org/10.3390/ijms19030833
- State medical cannabis laws. (2023, October 2). https://www.ncsl.org/health/state-medical-cannabis-laws
- Spindle, T. R., Bonn‐Miller, M. O., & Vandrey, R. G. (2019). Changing landscape of cannabis: novel products, formulations, and methods of administration. Current Opinion in Psychology, 30, 98–102. https://doi.org/10.1016/j.copsyc.2019.04.002
- Advocacy: Medical Cannabis CBD. (2023). Epilepsy Foundation. https://www.epilepsy.com/advocacy/priorities/medical-cannabis-cbd
- Shapiro, K. (2020, April 10). The Cannabis Industry Pays Tribute to Charlotte Figi, A Hero of The CBD Movement. Forbes. https://www.forbes.com/sites/katieshapiro/2020/04/10/the-cannabis-industry-pays-tribute-to-charlotte-figi-a-hero-of-the-cbd-movement/?sh=3d477f343829
- Raucci, U., Pietrafusa, N., Paolino, M. C., Di Nardo, G., Villa, M. P., Pavone, P., Terrin, G., Specchio, N., Striano, P., & Parisi, P. (2020). Cannabidiol treatment for refractory epilepsies in pediatrics. Frontiers in Pharmacology, 11. https://doi.org/10.3389/fphar.2020.586110
- Abu-Sawwa, R., & Stehling, C. (2020). Epidiolex (Cannabidiol) primer: Frequently asked questions for patients and caregivers. The Journal of Pediatric Pharmacology and Therapeutics, 25(1), 75–77. https://doi.org/10.5863/1551-6776-25.1.75
- Ryan, M. (2020). Cannabidiol in epilepsy: The indications and beyond. The Mental Health Clinician, 10(6), 317–325. https://doi.org/10.9740/mhc.2020.11.317
- Lawson, J. A., O’Brien, T., Graham, M., Renaud, E., Jones, D. P., Freeman, J. L., Lawn, N., & Martin, J. (2022). Expert advice for prescribing cannabis medicines for patients with epilepsy—drawn from the Australian clinical experience. British Journal of Clinical Pharmacology, 88(7), 3101–3113. https://doi.org/10.1111/bcp.15262
- Devinsky, O., Patel, A. D., Cross, J. H., Villanueva, V., Wirrell, E., Privitera, M., Greenwood, S. M., Roberts, C., Checketts, D., VanLandingham, K., & Zuberi, S. M. (2018). Effect of cannabidiol on drop seizures in the Lennox–Gastaut syndrome. The New England Journal of Medicine, 378(20), 1888–1897. https://doi.org/10.1056/nejmoa1714631
- Stith, S. S., Vigil, J. M., Brockelman, F., Keeling, K., & Hall, B. (2019). The Association between Cannabis Product Characteristics and Symptom Relief. Scientific Reports, 9(1). https://doi.org/10.1038/s41598-019-39462-1
- Perucca, E. (2017). Cannabinoids in the treatment of epilepsy: hard evidence at last? Journal of Epilepsy Research, 7(2), 61–76. https://doi.org/10.14581/jer.17012
- Filipiuc, L. E., Ababei, D. C., Alexa-Stratulat, T., Pricope, C. V., Bild, V., Ştefănescu, R., Stanciu, G. D., & Tamba, B. I. (2021). Major phytocannabinoids and their related compounds: Should we only search for drugs that act on cannabinoid receptors? Pharmaceutics, 13(11), 1823. https://doi.org/10.3390/pharmaceutics13111823
- Burns, P. B., Rohrich, R. J., & Chung, K. C. (2011). The levels of evidence and their role in Evidence-Based Medicine. Plastic and Reconstructive Surgery, 128(1), 305–310. https://doi.org/10.1097/prs.0b013e318219c171
- Rosenberg, E. C., Chamberland, S., Bazelot, M., Nebet, E. R., Wang, X., McKenzie, S., Jain, S., Greenhill, S., Wilson, M. A., Marley, N., Salah, A., Bailey, S., Patra, P. H., Rose, R., Chenouard, N., Sun, S. D., Jones, D. R., Buzsáki, G., Devinsky, O., . . . Tsien, R. W. (2023). Cannabidiol modulates excitatory-inhibitory ratio to counter hippocampal hyperactivity. Neuron, 111(8), 1282-1300.e8. https://doi.org/10.1016/j.neuron.2023.01.018
- Bih, C. I., Chen, T., Nunn, A. V., Bazelot, M., Dallas, M. L., & Whalley, B. J. (2015). Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics, 12(4), 699–730. https://doi.org/10.1007/s13311-015-0377-3
- Treiman, D. M. (2001). GABAergic mechanisms in epilepsy. Epilepsia, 42, 8–12. https://doi.org/10.1046/j.1528-1157.2001.042suppl.3008.x
- Silvestro, S., Mammana, S., Cavalli, E., Bramantı, P., & Mazzon, E. (2019). Use of cannabidiol in the treatment of epilepsy: efficacy and security in clinical trials. Molecules, 24(8), 1459. https://doi.org/10.3390/molecules24081459
- Barker‐Haliski, M., & White, H. S. (2015). Glutamatergic Mechanisms Associated with Seizures and Epilepsy. Cold Spring Harbor Perspectives in Medicine, 5(8), a022863. https://doi.org/10.1101/cshperspect.a022863
- Pretzsch, C. M., Freyberg, J., Voinescu, B., Lythgoe, D. J., Horder, J., Méndez, M. A., Wichers, R., Ajram, L., Ivin, G., Heasman, M., Edden, R. A., Williams, S., Murphy, D., Daly, E., & McAlonan, G. (2019). Effects of cannabidiol on brain excitation and inhibition systems; a randomised placebo-controlled single dose trial during magnetic resonance spectroscopy in adults with and without autism spectrum disorder. Neuropsychopharmacology, 44(8), 1398–1405. https://doi.org/10.1038/s41386-019-0333-8
- Cheung, K., Peiris, H. N., Wallace, G., Holland, O. J., & Mitchell, M. D. (2019). The Interplay between the Endocannabinoid System, Epilepsy and Cannabinoids. International Journal of Molecular Sciences, 20(23), 6079. https://doi.org/10.3390/ijms20236079
- Atalay, S., Jarocka-Karpowicz, I., & Skrzydlewska, E. (2019). Antioxidative and Anti-Inflammatory properties of cannabidiol. Antioxidants, 9(1), 21. https://doi.org/10.3390/antiox9010021
- Rosenberg, E. C., Tsien, R. W., Whalley, B. J., & Devinsky, O. (2015). Cannabinoids and epilepsy. Neurotherapeutics, 12(4), 747–768. https://doi.org/10.1007/s13311-015-0375-5
- Taalab, Y., Mohammed, W. F., Helmy, M., Othman, A. a. A., Darwish, M., Hassan, I., & Abbas, M. (2019). Cannabis Influences the Putative Cytokines-Related Pathway of Epilepsy among Egyptian Epileptic Patients. Brain Sciences, 9(12), 332. https://doi.org/10.3390/brainsci9120332
- Ross, H. R., Napier, I. A., & Connor, M. (2008). Inhibition of recombinant human T-type calcium channels by Δ9-Tetrahydrocannabinol and cannabidiol. Journal of Biological Chemistry, 283(23), 16124–16134. https://doi.org/10.1074/jbc.m707104200
- Martínez-Aguirre, C., Carmona-Cruz, F., Velasco, A. L., Velasco, F., Aguado-Carrillo, G., Cuéllar-Herrera, M., & Rocha, L. (2020). Cannabidiol acts at 5-HT1A receptors in the human brain: Relevance for treating temporal lobe epilepsy. Frontiers in Behavioral Neuroscience, 14. https://doi.org/10.3389/fnbeh.2020.611278
- Balachandran, P., ElSohly, M. A., & Hill, K. P. (2021). Cannabidiol Interactions with Medications, Illicit Substances, and Alcohol: A Comprehensive Review. Journal of General Internal Medicine, 36(7), 2074–2084. https://doi.org/10.1007/s11606-020-06504-8
- National Institute of Diabetes and Digestive and Kidney Diseases. (2023, February 16). Cannabidiol. LiverTox – NCBI Bookshelf. https://www.ncbi.nlm.nih.gov/books/NBK548890/
- Oberbarnscheidt, T., & Miller, N. S. (2020). The impact of cannabidiol on psychiatric and medical conditions. Journal of Clinical Medicine Research, 12(7), 393–403. https://doi.org/10.14740/jocmr4159
- Office of the Commissioner. (2019). What you should know about using cannabis, including CBD, when pregnant or breastfeeding. U.S. Food and Drug Administration. https://www.fda.gov/consumers/consumer-updates/what-you-should-know-about-using-cannabis-including-cbd-when-pregnant-or-breastfeeding
- Lachenmeier, D. W., Habel, S., Fischer, B., Herbi, F., Zerbe, Y., Bock, V., De Rezende, T. R., Walch, S. G., & Sproll, C. (2023). Are adverse effects of cannabidiol (CBD) products caused by tetrahydrocannabinol (THC) contamination? F1000Research, 8, 1394. https://doi.org/10.12688/f1000research.19931.6
- Pinto, J. S., & Martel, F. (2022). Effects of cannabidiol on appetite and body weight: a systematic review. Clinical Drug Investigation, 42(11), 909–919. https://doi.org/10.1007/s40261-022-01205-y
- Huestis, M. A., Solimini, R., Pichini, S., Pacifici, R., Carlier, J., & Busardò, F. P. (2019). Cannabidiol adverse effects and toxicity. Current Neuropharmacology, 17(10), 974–989. https://doi.org/10.2174/1570159×17666190603171901
- Hill, A., Mercier, P. P., Hill, T., Glyn, S., Jones, N., Yamasaki, Y., Futamura, T., Duncan, M., Stott, C., Stephens, G., Cm, W., & Whalley, B. (2012). Cannabidivarin is anticonvulsant in mouse and rat. British Journal of Pharmacology, 167(8), 1629–1642. https://doi.org/10.1111/j.1476-5381.2012.02207.x
- Grinspoon, P., MD. (2021). The endocannabinoid system: Essential and mysterious. Harvard Health. https://www.health.harvard.edu/blog/the-endocannabinoid-system-essential-and-mysterious-202108112569
- Balachandran, P., ElSohly, M. A., & Hill, K. P. (2021). Cannabidiol Interactions with Medications, Illicit Substances, and Alcohol: A Comprehensive Review. Journal of General Internal Medicine, 36(7), 2074–2084. https://doi.org/10.1007/s11606-020-06504-8
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click the Green MARK COMPLETE Button Below
To receive your certificate
