Course
Stress Cardiomyopathy
Course Highlights
- In this Stress Cardiomyopathy course, we will learn about the signs and symptoms of stress cardiomyopathy.
- You’ll also learn the risk factors for stress cardiomyopathy.
- You’ll leave this course with a broader understanding of the pathophysiology of stress cardiomyopathy.
About
Contact Hours Awarded: 3
Course By:
Keith Wemple, BSN, R.N., CCRN, CMC
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction
“They died of a broken heart”. You may have heard this phrase before, but did you know that “broken heart syndrome” is a real thing? In the medical community, we call it stress cardiomyopathy. Stress cardiomyopathy is a unique disease in that it can be triggered by a major emotional stressor, or a “broken heart”. Stress cardiomyopathy disguises itself as a heart attack and can be confused with other cardiac conditions.
Stress cardiomyopathy is fairly rare, with an incidence of about 0.3% overall and 1-2% of patients with acute coronary syndrome, although experts believe the actual numbers may be higher (1). Although it is rare, stress cardiomyopathy can have serious consequences often requiring hospitalization and may lead to critical illness or even death.
But how does a broken heart lead to a potentially fatal condition? How do you diagnose stress cardiomyopathy if it mimics other conditions? And how do you treat it? This course will answer these questions (and pose some of our own) to arm you with the knowledge you need to manage “broken heart syndrome”.

Self Quiz
Ask yourself...
- What do you know about stress cardiomyopathy already?
- Have you ever cared for a patient with stress cardiomyopathy? How did the patient recover?
Definition
First, let us start with a basic definition of stress cardiomyopathy. This isn’t as easy as it sounds, because stress cardiomyopathy goes by many names. As alluded to in the introduction, stress cardiomyopathy is known colloquially as broken heart syndrome. It also has several different names used by medical professionals, including Takotsubo cardiomyopathy, apical ballooning syndrome, and stress cardiomyopathy.
Whatever name you use, stress cardiomyopathy is defined as an acute reversible condition of the heart muscle that causes regional systolic left ventricular dysfunction (1). In plain English, stress cardiomyopathy is the abnormal movement of part of the left ventricle that causes heart failure. The base of the heart contracts while the apex does not, forcing blood down into the apex instead of out to the other organs of the body.
The apex balloons out because of the extra blood being forced into it, hence the name apical ballooning syndrome. The name Takotsubo (pronounced tack-uht-sue-bow) comes from its discovery in Japan where they thought the left ventricle in this disease looked like an octopus trap (takotsubo in Japanese).

Self Quiz
Ask yourself...
- Looking at the picture above, what is happening with the left ventricle?
- What consequences could this have for the patient?
- What names have you heard for stress cardiomyopathy?
Etiology
Now that we know what stress cardiomyopathy is, let us find out how a “broken heart” can lead to this acute left ventricular dysfunction. Stress cardiomyopathy can be triggered by either an emotional or physical stressor. Emotional stressors that can trigger this condition include the death of a loved one, assault, great financial loss, or any major stressor especially those creating a sense of danger or desperation (1).
Physical stressors that may trigger stress cardiomyopathy include acute critical illness, surgery, sepsis, stroke, or other major physical stressors (1). Stress cardiomyopathy seems to be triggered by physical and emotional triggers roughly equally (5).
Stress cardiomyopathy has two distinct presentations depending on the cause. In primary stress cardiomyopathy, the stress cardiomyopathy itself produces the symptoms that lead a person to seek treatment (1). For example, a patient goes to the emergency room with shortness of breath from stress cardiomyopathy after their father’s funeral. In secondary stress cardiomyopathy, the major physical stressor that triggers the stress cardiomyopathy is the primary reason for treatment (1). Symptoms of stress cardiomyopathy usually develop 2-5 days after the emotional stressor.
Secondary stress cardiomyopathy is often harder to diagnose because the initial health problem could also cause the symptoms of stress cardiomyopathy. For example, if a person presents with sepsis and develops stress cardiomyopathy they could have hypotension from sepsis just as easily as from stress cardiomyopathy.


Self Quiz
Ask yourself...
- How could you differentiate worsening sepsis from a new diagnosis of stress cardiomyopathy?
- What conditions that you typically treat could trigger stress cardiomyopathy?
- What stressors can you think of that would create “a sense of danger or desperation”?
Pathophysiology
The physical or emotional offending stressor triggers a sympathetic response in the body. This sympathetic response causes a release of catecholamines, such as epinephrine and norepinephrine (3). These catecholamines cause the release of inflammatory cytokines in the blood and trigger smooth muscle vasoconstriction (3). The heart is made of smooth muscle, so it is affected by these high levels of catecholamines.
The apex of the heart is more sensitive to catecholamines, so it has an exaggerated response to the increased levels of epinephrine and norepinephrine (3). The apex gets “stunned” by the high levels of catecholamines, while the rest of the heart squeezes more strongly (3). This explains why the base of the heart constricts down more than the apex, causing the hallmark trapping of blood in the apex.
Major stress also triggers the release of inflammatory cytokines and free radicals (3). The increased levels of inflammatory substances injure the heart muscle and blood vessels (3). Damage to the heart muscle helps explain some of the heart failure in stress cardiomyopathy. The injured blood vessels then have an abnormal response to the higher levels of circulating catecholamines.
This causes blood vessels to spasm and increases afterload on the left ventricle (3). The increased workload on the left ventricle compounds with the ineffective apex, and damaged heart muscle to cause the acute heart failure of stress cardiomyopathy (3).
The coronary arteries are also affected by the high levels of catecholamines and inflammatory markers. The coronary arteries also spasm, causing chest pain and a syndrome of findings like a heart attack (3). Remember, the coronary arteries are what supply blood and oxygen to the muscles of the heart. The lack of blood flow through the spasmed coronaries adds to the dysfunction of the heart by limiting blood flow to the struggling heart muscle (3).
While most stress cardiomyopathy cases present with the usual apical ballooning pattern, there are other variants. 10-20% of cases of stress cardiomyopathy have a midventricular ballooning pattern, where the “pinch point” is midway down the ventricle rather than near the apex (1). This leads to more trapping of blood and worse cardiogenic shock (1).

Self Quiz
Ask yourself...
- What other problems could arise from this pathophysiology?
- What conditions might make a person more likely to develop stress cardiomyopathy after a significant stressor?
- What therapies might help treat this pathophysiology?
Risk Factors
Not every person who experiences a significant stressor experiences this cascade of problems, otherwise we would all have a story of stress cardiomyopathy. So, let’s find out what makes a person at higher risk of developing stress cardiomyopathy.
The prototypical stress cardiomyopathy patient is a post-menopausal woman who has experienced major emotional stress in the last 5 days (1). The cardiovascular changes post-menopause mimic much of the pathophysiology of stress cardiomyopathy. Menopause leads to increased stiffness and dysfunction of the blood vessels, increased free radicals, and a mild decline in heart function (4). The similarity in postmenopausal changes and the pathophysiology of stress cardiomyopathy explains why these women are at higher risk.
Diabetes mellitus is another risk factor for stress cardiomyopathy and is present in up to 25% of cases (1). Diabetes causes changes in the blood vessels which makes them more likely to respond pathologically to increased catecholamines and inflammatory markers (1). Diabetes is also a known risk factor for cardiac disease in general, so they are more likely to have some level of pre-existing cardiovascular dysfunction (3). Being at greater risk and starting from a lower level of cardiac function explains why diabetic patients who experience stress cardiomyopathy have a higher mortality (1).
Cannabis use disorder is also linked to a higher risk of developing stress cardiomyopathy and a higher rate of cardiac arrest when stress cardiomyopathy does occur (1). Cannabinoids affect central nervous system control of cardiac function, reducing heart muscle contractility, and may be related to vascular dysfunction (1). This lowers the threshold for patients experiencing major stress to enter the realm of stress cardiomyopathy. These changes also compound with the left ventricular and vascular dysfunction caused by stress cardiomyopathy, resulting in higher rates of cardiac arrest (1).
Finally, chronic stress and psychiatric disorders can increase the risk of developing stress cardiomyopathy (1). Stress and anxiety produce similar effects to the pathophysiology of stress cardiomyopathy. Catecholamine and cortisol levels increase which over time depress heart function (3). As many as 43% of stress cardiomyopathy patients have been treated for a psychiatric disorder (3).

Self Quiz
Ask yourself...
- How often do you see patients with one or more of these risk factors?
- Remembering pathophysiology, are there additional risk factors you think may worsen stress cardiomyopathy?
- Is there a condition or type of patient where these risk factors might start to “stack up”?
Signs and Symptoms
The signs and symptoms of stress cardiomyopathy initially are similar to other acute cardiac conditions. This includes symptoms of chest pain, dyspnea, nausea, palpitations, and syncope (3). Spasming of the coronary arteries during stress cardiomyopathy causes a lack of blood flow to the heart muscle, and therefore ischemic pain much like a heart attack. Just like in a true myocardial infarction, a disturbance in the blood flow to the heart can cause arrhythmias to develop. The drop in cardiac output from stress cardiomyopathy leads to poor perfusion of other organs leading to the symptoms of nausea, dyspnea, and syncope.
ECG changes are often present, including ST depression or elevation and T wave inversion (1). Again, these are similar to changes seen during myocardial ischemia and are caused by the spasming of the coronary arteries. On laboratory analysis, patients typically have elevated troponin, brain natriuretic peptide (BNP), and inflammatory markers (5). On echocardiogram, stress cardiomyopathy patients will demonstrate the classic regional wall motion abnormalities with apical ballooning (1).
Returning to the pathophysiology of stress cardiomyopathy, these symptoms make sense. Heart failure often produces symptoms of dyspnea, palpitations, nausea, and syncope. Additionally, coronary vasospasm and increased stress on the heart from stress cardiomyopathy can easily produce chest pain and dyspnea.

Self Quiz
Ask yourself...
- What other conditions have similar symptoms to stress cardiomyopathy?
- How do you think you could differentiate between these conditions?
- What treatments would be appropriate to control the symptoms of stress cardiomyopathy?
Diagnosis
As you probably have already realized, stress cardiomyopathy presents very similarly to acute coronary syndrome or a heart attack. A heart attack also presents chest pain, shortness of breath, ECG changes, and an elevated troponin. As a result, the diagnosis of stress cardiomyopathy is often one of exclusion (1). Initial findings of chest pain, elevated troponin, and ECG changes are shared between stress cardiomyopathy and acute myocardial infarction.
There are, however, a few findings on the initial workup that would point more towards stress cardiomyopathy. Patients with stress cardiomyopathy more often present with an emotional trigger to their symptoms, while patients with acute coronary syndrome often have no clear trigger (5).
This means interviewing the patient on the causes of their symptoms is important. Also, patients with stress cardiomyopathy are more likely to present with higher initial troponin and brain natriuretic peptide levels, while those with acute coronary syndrome present with a higher creatinine kinase (5). Finally, ST segment depression is less common and T-wave inversion is more common in patients experiencing stress cardiomyopathy (5).
To differentiate the syndromes, either a coronary angiogram or echocardiogram is often performed (1). As a reminder, a coronary angiogram is a procedure where catheters are inserted into a patient’s artery, and dye is injected into the coronary arteries to assess blood flow to the heart muscle. If you want to learn more about coronary angiograms, we have a course for that (shameless plug).
On angiogram, a patient with stress cardiomyopathy often has no acute blockage of the coronary arteries, while a patient with acute myocardial infarction will (1). A patient can have an acute blockage of a coronary artery on top of stress cardiomyopathy, but this is very rare (1). A ventriculogram can also be performed at the time of a coronary angiogram to help diagnose the condition. A ventriculogram involves injecting dye directly into the left ventricle to visualize the movement of blood within the ventricle (1). This will show the typical apical ballooning pattern of stress cardiomyopathy.
Echocardiography is one of the primary ways to differentiate stress cardiomyopathy from other heart conditions, The echocardiogram will show regional wall motion abnormalities that affect the entire circumference of the heart just above the apex (1). This area of the heart muscle does not contract well, producing the classic octopus trap or apical ballooning appearance of the left ventricle.
By contrast, in acute coronary syndrome, the heart will show regional wall motion abnormalities in an isolated area of the heart, rather than the entire circumference of the ventricle (1). The area of abnormal movement in acute coronary syndrome is reflective of the artery that is blocked. For example, if the left anterior descending artery is blocked the anterior wall of the left ventricle would show abnormal movement.
Image: ECG of Takotsubo cardiomyopathy
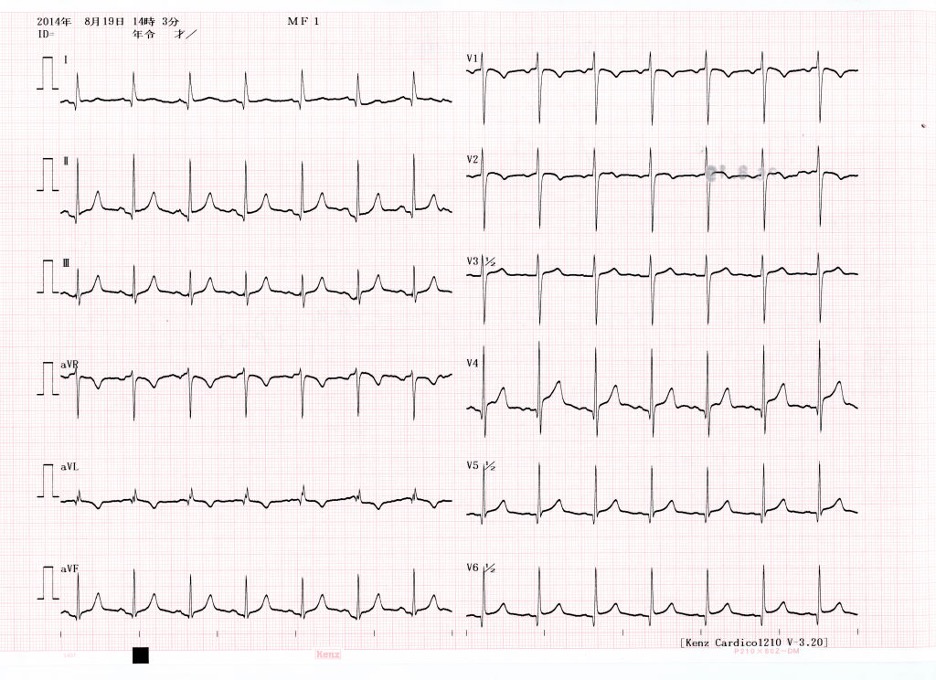 (8)
(8)

Self Quiz
Ask yourself...
- Does your workplace have a cardiac cath lab where an angiogram could be performed?
- If you suspected stress cardiomyopathy, how would you have an echo performed quickly for a patient?
- How confident are you in interpreting ECG and echo reports?
Epidemiology
Stress cardiomyopathy is preceded by a major stressor in over 90% of cases, with about half arising from a physical stressor and the other from an emotional stressor (5). The physical stressors commonly reported include surgery, sepsis, stroke, trauma, or acute critical illness – caused by pulmonary embolism, sepsis, etc., (1,5).
Emotional stressors that trigger stress cardiomyopathy include the death of a loved one, major financial stress – like the loss of a job, assault, or another emotional stressor that causes a sense of loss and desperation (1). Up to 95% of patients with stress cardiomyopathy are female. These women are most often postmenopausal with an average age of 67 (5). A coexisting diagnosis of cancer, COPD, asthma, neurologic or psychiatric disorder is more common in stress cardiomyopathy than acute coronary disease (5).


Self Quiz
Ask yourself...
- How often do you care for patients fitting this picture?
- Why do you think a coexisting diagnosis of cancer, COPD, or asthma is more common?
Treatment
Because stress cardiomyopathy is a response to a physical or emotional stressor, it often resolves within a few weeks (1). As a result, treatment is supportive until the patient recovers. Patients merely need support to keep their vital organs perfused until the disease resolves itself.
Careful monitoring is an important part of the treatment of stress cardiomyopathy. ECG monitoring is recommended for 48 hours, or until the patient has been stabilized, in order to detect arrhythmias (1). Hemodynamics must be closely monitored, which may require the placement of an arterial line or pulmonary artery catheter depending on the severity of the disease (7). Echocardiograms should be performed with initial diagnosis, with a major worsening of the disease or when signs of complications arise (1).
Supportive care for heart failure is the main focus of treatment. This includes supporting hemodynamics and relieving pulmonary congestion caused by heart failure. For patients with pulmonary edema and adequate blood pressure and cardiac output, nitroglycerine and diuretics are recommended (1).
In acute heart failure, the heart is unable to move enough blood forward resulting in an increased preload on the heart and a backup of fluid (7). Nitroglycerine helps dilate veins and reduces preload on the heart. Diuretics, such as furosemide, also reduce preload on the heart and get rid of excess fluid which may accumulate in the lungs (1). Less fluid going into the heart means less work that the heart has to do and less fluid which can build up in other organs.
For patients with high blood pressure, beta-blockers such as metoprolol or carvedilol are helpful (1). High blood pressure increases the resistance to blood leaving the heart and can worsen heart failure (7). Beta-blockers help reduce blood pressure and also slow the heart rate so that the heart can fill and pump more effectively (6). Beta-blockers also have the advantage of reducing the risk of arrhythmias (7). Beta blockers must be used carefully in acute heart failure, however, as they further depress the contractility of the heart muscle.
If the patient has hypotension, an echo must be performed to guide treatment. The abnormal movement of the heart in stress cardiomyopathy can sometimes obstruct blood flow out of the left ventricle (1). If there is no obstruction, inotropes such as dobutamine or milrinone should be used to improve cardiac output (1). Positive inotropes encourage stronger contractions from the weaker heart so that more blood can be delivered to the rest of the body.
If there is an outflow tract obstruction, inotropes should be avoided and low-dose beta-blockers and vasopressors, such as phenylephrine, should be used instead (1). Beta-blockers and phenylephrine cause changes in the heart and blood vessels which relieve the obstruction and allow more blood to be delivered into the aorta (1).
In either case, if a patient is unresponsive to initial treatment of cardiogenic shock mechanical circulatory support may be needed. Intra-aortic balloon pump counter pulsation, and left ventricular support devices such as Impella, or ECMO are commonly utilized. These methods help mechanically improve cardiac output beyond what the heart can achieve. Which device is appropriate depends upon the severity of the condition and availability of devices.
Below is a nice flowchart from the American College of Cardiology detailing the treatment of stress cardiomyopathy based on presentation (1).
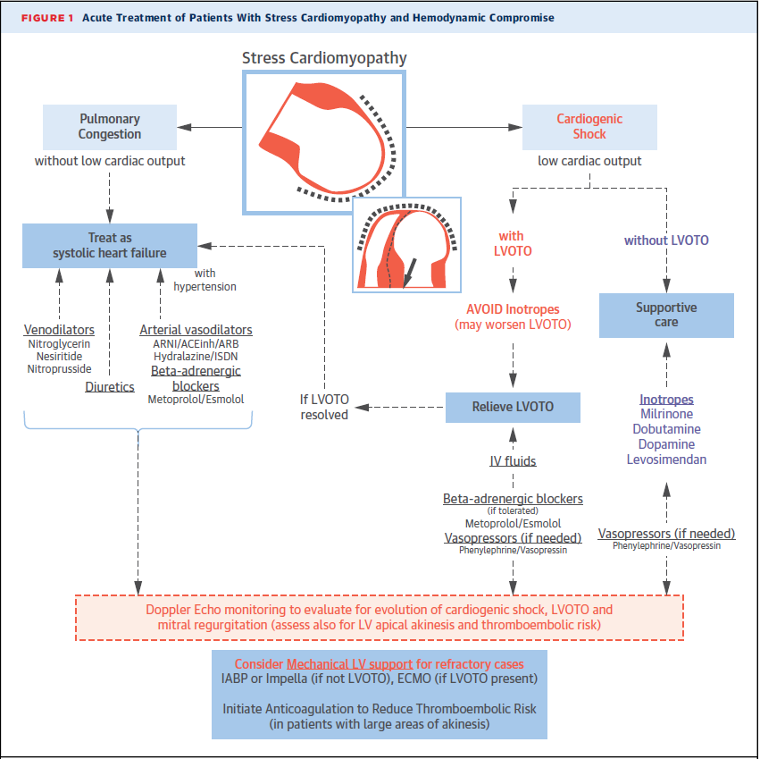
(1)

Self Quiz
Ask yourself...
- What is the pharmacology of the medications discussed? (Beta blockers, inotropes, phenylephrine, nitroglycerine)
- The ACC recommends mechanical support in severe stress cardiomyopathy. Does your workplace have the capability to run mechanical cardiac support devices?
- If not, where is the closest location you could transfer this patient that could provide mechanical support?
- How would treatment differ if stress cardiomyopathy was misdiagnosed as a myocardial infarction?
Complications
Stress cardiomyopathy can range from mild and managed at home to critical illness requiring mechanical support. A key aspect of the disease process is the development of complications. The most serious complications of stress cardiomyopathy are cardiogenic shock, left ventricular outflow tract obstruction, arrhythmias, and thromboembolism (1).
Acute heart failure is part of the disease process of stress cardiomyopathy but may worsen into full-blown cardiogenic shock. Stress cardiomyopathy becomes cardiogenic shock when the patient becomes hypotensive to the point that they require inotropes or have evidence of organ dysfunction (7).
Evidence of organ dysfunction from poor cardiac output includes findings such as oliguria, elevated lactate, elevated liver enzymes, or decreased SvO2 (7). Treatment of this complication involves inotropes, diuresis, and potentially mechanical circulatory support, as outlined in the treatment section.
Left ventricular outflow tract obstruction is an important complication to monitor for, as it guides the treatment of stress cardiomyopathy. Left ventricular outflow tract obstruction (LVOTO) is when the abnormal movement of the heart in stress cardiomyopathy blocks blood flow out of the left ventricle into the aorta (1). Essentially, the base of the heart contracts in a way where the wall of the heart or the mitral valve itself moves in front of the aortic valve and impedes blood flow into the aorta.
LVOTO is diagnosed with an echocardiogram, where the abnormal movement of the heart and outflow tract obstruction can be visualized (1). This complication is serious, as it further reduces cardiac output and cannot be treated in the same way as typical cardiogenic shock. Increasing the contractility of the heart with inotropes is helpful in cardiogenic shock but can be harmful in LVOTO. Increasing contractility in a patient with LVOTO can cause the abnormally moving heart muscle to obstruct more blood flow, worsening the obstruction (1). Treatment instead involves using vasopressors and beta blockers to reduce obstruction.
Arrhythmias are common in stress cardiomyopathy, affecting about 1 of every 4 patients (1). Atrial fibrillation is most common and can worsen an already low cardiac output, as well as increase the risk of thrombus (1). Ventricular arrhythmias occur in up to 9% of cases and can be life-threatening (1). Because of this risk, patients with symptomatic stress cardiomyopathy should have continuous ECG monitoring (1). Any arrhythmias that do occur are treated with anti-arrhythmic medications as usual. This typically includes medications such as amiodarone, beta-blockers or lidocaine. If the patient is unstable because of an arrhythmia a cardioversion may be necessary.
A thrombus can form in the left ventricle during stress cardiomyopathy, because of the trapping of blood in the apex; this complication typically develops in the first 5 days of the disease and is present in up to 9% of cases (1). Echocardiography is used to monitor the formation of a thrombus. If a thrombus does occur, the patient is treated with anticoagulation for at least 2 weeks or until the thrombus resolves (1). A thrombus in the left ventricle is a serious complication because it can lead to a stroke or embolus in any of the other organs (1).

Self Quiz
Ask yourself...
- Have you ever seen one of these complications in a patient with acute heart failure?
- If so, how was it diagnosed and treated?
- What anticoagulants and antiarrhythmics do you typically see prescribed?
Self-Management
Because stress cardiomyopathy has a self-limiting disease process, it is possible for patients with mild disease to self-manage their recovery. Mild cases of stress cardiomyopathy can be treated with a short course of anticoagulation (typically aspirin), which can be self-managed by the patient (6).
Short-term use of beta blockers is also sometimes used to treat stress cardiomyopathy outside the hospital (6). Anytime patients are prescribed medications to self-manage recovery they should be well educated on the side effects of the medications and when to stop taking the medications.
Some patients with mild disease can even recover with no medical therapy at all. If symptoms have subsided and the risk of arrhythmias has been explored, it is reasonable to allow patients to recover at home. This involves having patients not exert themselves, monitoring for the return of symptoms, and avoiding the stressor that triggered the initial stress cardiomyopathy (6). Again, educating the patient on what symptoms to watch for and report is imperative. If stress cardiomyopathy is triggered by an emotional stressor, collaborating with mental health professionals may be helpful.

Self Quiz
Ask yourself...
- What education strategies have you found helpful for patients?
- What specific symptoms should patients monitor for?
- What mental health resources are available in your community?
Research Findings
To date, there have been no randomized controlled trials on the treatment of stress cardiomyopathy. Adenosine has been shown to promote a rapid improvement in regional wall motion abnormalities caused by stress cardiomyopathy (6). The reason for this is that adenosine reduces sympathetic stimulation which helps alleviate the excess of catecholamines believed to cause stress cardiomyopathy (6).
A randomized controlled study has been proposed that would evaluate the use of adenosine and dipyridamole to treat stress cardiomyopathy (6). Dipyridamole reduces the reuptake of adenosine so that it is not cleared too quickly and would make adenosine therapy more effective (6). This would be the first randomized controlled study of medical therapy that directly targets the pathophysiology of stress cardiomyopathy.
A study of risk factors and presentation of stress cardiomyopathy was conducted to develop a scoring tool to aid in diagnosis. This InterTAK Diagnostic Score gives some points for the presence of female sex, emotional trigger, physical trigger, absence of ST segment depression, psychiatric disorder, neurologic disorder, and QTc prolongation based on their correlation with stress cardiomyopathy (5). A total score greater than 50 suggests a diagnosis of stress cardiomyopathy with a 95% specificity (5).

Self Quiz
Ask yourself...
- Why do you think there has never been a randomized controlled trial looking at the treatment of stress cardiomyopathy?
- What other research might aid in the diagnosis and treatment of stress cardiomyopathy?
- What might be potential side effects or complications of adenosine therapy?
- What information from this course can you apply to your nursing practice?
- Are the staff at your workplace knowledgeable on stress cardiomyopathy?
- If not, how could you share this message or incorporate your knowledge into the practice at your workplace?
Case Study
Jackie Smith, a 70-year-old female patient, reports to the emergency department with chest pain and shortness of breath accompanied by her daughter. Jackie has a history of diabetes mellitus, hypertension, generalized anxiety disorder, and arthritis. You assess the patient and find that she has 7/10 crushing chest pain, difficulty breathing, and is tearful. Jackie is tachycardic with a regular rate and rhythm, tachypneic with diminished lung sounds in her bilateral lung bases, no obvious edema or trauma, and her skin is pink with a capillary refill of less than 3 seconds. Jackie’s vitals are BP 144/80, HR 118, RR 24, SpO2 88% on room air, temp 36.7 C.
- What tests do you want ordered to diagnose Jackie’s condition?
- What risk factors does Jackie have for stress cardiomyopathy?
- Are there any treatments you want to start now?
You administer 2LPM of oxygen via nasal cannula and ask the provider to order nitroglycerine for Jackie’s chest pain. The provider also orders an ECG and lab work. While caring for Jackie, her daughter begins crying and explains that her father (Jackie’s husband) passed away earlier this week, and she can’t bear to lose her mother too. You comfort Jackie’s daughter while the ECG is performed, and labs are drawn. The ECG shows T wave inversions and premature ventricular complexes (PVCs). The labs show an elevated troponin, BNP, WBC, and a hemoglobin of 11.
- Based on the assessments so far what do you think Jackie’s diagnosis is?
- What are the differential diagnoses?
- How can you distinguish this diagnosis from its differentials?
Jackie’s chest pain and hypoxia improve with your treatment. She now rates her chest pain 1/10 and her SpO2 reads 97% on 2L via nasal cannula. She is then sent to the cardiac cath lab for a coronary angiogram to rule out acute myocardial infarction. The procedure is uneventful. The coronary angiogram shows only mild, chronic disease in the coronary arteries with no acute blockage. Jackie is admitted to the cardiac unit for further workup and monitoring.
- What test would you like next to diagnose Jackie’s condition?
- What monitoring should be instituted?
Shortly after arrival to the cardiac unit Jackie’s vitals are BP 86/50, HR 122, SpO2 94% on 6L via nasal cannula, Temp 36.4 C, 2/10 pain in her chest. Her ECG shows sinus tachycardia with frequent PVCs. An echocardiogram is performed urgently, and Jackie is diagnosed with stress cardiomyopathy and a reduced ejection fraction down to 25%.
- What findings on the echo would lead to the diagnosis of stress cardiomyopathy?
- What treatment is appropriate for Jackie given her vitals and diagnosis?
Jackie was started on a dobutamine infusion and transferred to the intensive care unit. On arrival at the ICU, her respiratory status worsened, and a chest x-ray was performed. The chest x-ray reveals pulmonary edema. Her urine output has decreased, and her labs show elevated creatinine, BUN, AST, and ALT.
- What is the cause of Jackie’s pulmonary edema?
- What is an appropriate treatment for her pulmonary edema?
- What is causing the increase in her renal and liver function tests?
Jackie is started on BiPAP and given furosemide for diuresis. Her dobutamine infusion is up-titrated due to evidence of poor organ perfusion. Overnight Jackie’s condition improves. Her urine output has improved, and she feels less short of breath. Her vitals are now: BP 100/80, HR 106, Temp 36.5, 0/10 pain, and SpO2 99% on BiPAP with settings 16/6 cmH2O and FiO2 60%.
- Besides the improvement in her symptoms, what tests could be performed to assess Jackie’s progress?
After another day of recovery, Jackie continues to improve. She is weaned off her dobutamine infusion and BiPAP and remains vitally stable. She is now deemed appropriate to be transferred out of the ICU. Her vitals before transfer are BP 116/70, HR 88, RR 20, SpO2 98% on 2L via nasal cannula, Temp 36.8 C, and 0/10 pain. A repeat echo is done before she is transferred out of the unit, which shows an improved ejection fraction of 45% but a new thrombus in the left ventricle.
- What treatment is appropriate for the new LV thrombus?
- What other complications should Jackie be monitored for?
Jackie was started on apixaban as the anticoagulant for her LV thrombus and transferred to the cardiac unit. Jackie is monitored for another 2 days and continues to recover well. She no longer requires oxygen, is vitally stable, and is able to ambulate around the unit. She is set to be discharged from the hospital this afternoon.
- What education do you want to provide Jackie before she is discharged?
- What symptoms should Jackie monitor for at home?
Jackie teaches back that she must watch for symptoms of shortness of breath, chest pain, changes in her mental status, or inability to move her extremities. She repeats her medication schedule and will continue her medications until a follow-up with her doctor next week.
- What actions by Jackie’s healthcare team helped her successful treatment?
- How might Jackie’s story have ended differently if the correct diagnosis had not been made?
Conclusion
In summary, stress cardiomyopathy (or broken heart syndrome) is an acute, reversible form of cardiomyopathy triggered by an emotional or physical stressor. The stressor leads to a large catecholamine and inflammatory response that causes abnormal movement of the heart and ballooning of the apex.
The symptoms mimic those of acute coronary syndrome but can be differentiated by small differences in presentation, echocardiogram, and coronary angiography. Treatment is supportive care based on the severity of symptoms, ranging from self-managed recovery to mechanical circulatory support.
Hopefully, by learning more about the disease process of stress cardiomyopathy, how to diagnose it, and how to treat it will aid you in your nursing practice. While it is a relatively rare condition, it can have serious consequences. The best way to prevent stress cardiomyopathy from becoming a critical illness is to know how to recognize it early and treat it appropriately. Armed with the knowledge from this course, you can go out and save someone from their broken heart.
References + Disclaimer
- Medina de Chazal, H, Del Buono, M, Keyser-Marcus, L. et al. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018 Oct, 72 (16) 1955–1971.https://doi.org/10.1016/j.jacc.2018.07.072
- Fish M. What is takotsubo (broken heart syndrome)? Heart Foundation NZ. 2024. Accessed June 19, 2024. https://www.heartfoundation.org.nz/your-heart/heart-conditions/takotsubo-cardiomyopathy.
- Al Houri HN, Jomaa S, Jabra M, Alhouri AN, Latifeh Y. Pathophysiology of stress cardiomyopathy: A comprehensive literature review. Ann Med Surg (Lond). 2022 Sep 15; 82:104671. doi: 10.1016/j.amsu.2022.104671. PMID: 36268377; PMCID: PMC9577654.
- Nair AR, Pillai AJ, Nair N. Cardiovascular Changes in Menopause. Curr Cardiol Rev. 2021;17(4): e230421187681. doi: 10.2174/1573403X16666201106141811. PMID: 33155924; PMCID: PMC8762155.
- Ghadri JR, Cammann VL, Jurisic S, Seifert B, Napp LC, Diekmann J, Bataiosu DR, D’Ascenzo F, Ding KJ, Sarcon A, Kazemian E, Birri T, Ruschitzka F, Lüscher TF, Templin C; InterTAK co-investigators. A novel clinical score (InterTAK Diagnostic Score) to differentiate takotsubo syndrome from acute coronary syndrome: results from the International Takotsubo Registry. Eur J Heart Fail. 2017 Aug;19(8):1036-1042. doi: 10.1002/ejhf.683. Epub 2016 Dec 7. PMID: 27928880.
- Madias JE. Takotsubo Cardiomyopathy: Current Treatment. J Clin Med. 2021 Aug 2;10(15):3440. doi: 10.3390/jcm10153440. PMID: 34362223; PMCID: PMC8347171.
- Sarma D, Jentzer JC. Cardiogenic Shock: Pathogenesis, Classification, and Management. Crit Care Clin. 2024 Jan;40(1):37-56. doi: 10.1016/j.ccc.2023.05.001. Epub 2023 Jul 6. PMID: 37973356.
- Med Chaos [Wiki Commons Image]. 2014. ECG of Takotsubo cardiomyopathy. Creative Commons Attribution-Share Alike 4.0 International license; CC-BY-SA-4.0. https://commons.wikimedia.org/wiki/File:ECG_of_Takotsubo_cardiomyopathy.jpg
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click the Green MARK COMPLETE Button Below
To receive your certificate
