Course
Tirzepatide for Type 2 Diabetes and Weight Management
Course Highlights
- In this course on Tirzepatide for Type 2 Diabetes and Weight Management you will understand the drug classification of tirzepatide.
- You’ll also learn to analyze the indications of usage for tirzepatide in diabetes and weight loss management.
- You’ll also be able to explain safe prescribing practices for this medication and important considerations.
About
Contact Hours Awarded: 1
Course By:
Amy White
RN-MSN
Begin Now
Read Course | Complete Survey | Claim Credit
➀ Read and Learn
The following course content
Introduction
The emergence of the drug tirzepatide is becoming more popular and widespread and is being utilized among those with diabetes and also those who desire to lose weight. It is one of the newest diabetic drugs given by injection that also triggers dramatic weight loss in those who use the injections.
The U.S. Food and Drug Administration (FDA) approved tirzepatide in 2022 for individuals with diabetes, particularly Type 2 Diabetes. The FDA officials have not approved tirzepatide yet for weight loss, but they are currently tracking the medication and may have a recommendation for its approval by the end of this year. Clinical trials have shown that individuals with an elevated body mass index (BMI) and who did not have diabetes lost a considerable amount of weight when they received tirzepatide (1).
Advanced Practice Registered Nurses (APRNs) need to understand how to safely prescribe tirzepatide and the reasoning as to why it causes weight loss for specific individuals.
Drug Classification
Tirzepatide is part of a class of medications called glucose-dependent insulin tropic polypeptide (GIP) receptor and glucagon-like peptide-1 (GLP-1) receptor agonists. It comprises a 39 amino acid linear synthetic peptide conjugate to selective receptor agonists in preclinical and clinical trials.
Tirzepatide is used for treating Type II diabetes in adults as an adjunct to diet and exercise. It is also used for weight loss in some individuals and has gained increased attention as a new therapeutic agent for glycemic and weight control.
Social media has had a significant influence and increased the desire to use tirzepatide, and while individual results vary, the weight loss in adults ranged from 12 – 25 pounds.
Online pharmacies, diet clinics, and medical spas are implementing thousands of ads on social media to capitalize on a surge of interest in the drug.

Self Quiz
Ask yourself...
- Why has there seemed to be an increase in patients requesting this medication? What other medicines intended for type 2 diabetes are also being used for weight loss management?
- What are the ethical considerations regarding marketing this drug for weight loss when its primary use is for type 2 diabetes? Could this impact supply and costs?
Indications of Usage
The use of tirzepatide is being used for both Type II diabetes and weight control in certain patients. It has been a game changer for people living with Type II diabetes. The drug’s primary use is as an adjunct to diet and exercise to improve glycemic control in adults with diabetes.
The drug has also proven beneficial for weight loss in patients experiencing obesity, and those who are taking the highest dosage have shared a body weight reduction of 15.7% (2). Tirzepatide is an injectable prescription medication used together with diet and exercise, and it is not yet known if it can be used safely with patients who have had pancreatitis.
It is important to remember that it is not to be used for patients with Type I diabetes, but it is safe for Type II diabetic patients. Also, the safety of tirzepatide has yet to be discovered for children and those under 18; therefore, the medication should not be used for this age group.
In studies conducted with or without diabetic medicines, 75% – 90% of patients taking tirzepatide reached an overall A1C of less than 7% with an average starting A1C of 7.9 – 8.6% across the following dosages – 5mg, 10mg, and 15mg. The study results were measured at weeks 40 and 52 (3).

Self Quiz
Ask yourself...
- What dietary and activity recommendations can you provide to patients using tirzepatide for weight loss?
- Is this drug intended for those who want to lose 5-10 pounds?
Use of Tirzepatide with Diabetic Patients
Tirzepatide can be used for patients with Type II diabetes in combination with a diabetic-friendly diet and exercise. The drug works by lowering the patient's overall blood sugar and also improves the A1C results of patients over some time. The injection has been approved by the FDA to treat Type II diabetes and is administered once weekly (4).
It is considered the first in a new class of medications – a dual glucose-dependent insulin tropic polypeptide (GIP) and glucagon-like-peptide-1 (GLP-1) receptor antagonist. The mechanism of how it works mimics two gut hormones (GIP and GLP-1). These hormones are essential in how patients digest food and regulate blood glucose after meals. The hormones also play a role in making individuals feel fuller and curb specific food cravings.
The provider can prescribe tirzepatide before attempting other diabetic medications if a patient has a BMI of 30 or greater or 27 or greater with weight-related conditions and if the drug is combined with a personalized weight loss plan that addresses physical activity, nutrition, and lifestyle changes.
However, due to the cost and some insurance companies not covering the injection unless the patient has both diabetes and obesity, the provider must carefully consider prescribing this medication.

Case Study
The patient states this 'miracle drug' is worth paying for out of pocket!
Jeff Capron, a 53-year-old Boonville, New York, web developer, started taking tirzepatide in December 2022. His friend had reported good results with the medication, so Jeff looked into the research studies behind it and then spoke with his primary physician.
The physician said, "Yeah, let's give it a shot," even though he did not have much experience with it. The physician did not have an opinion one way or the other than looking at the data set and seeing no reason why they could not try it.
Jeff's hemoglobin A1C went from 10.1% to 6% in 3 months, which was very promising. "I never had that kind of experience with any medication for diabetes." There is a range in how much A1C reduction people experience with tirzepatide, but many people taking it can get their A1C under 7% — an ideal goal for people with Type 2 diabetes.
Jeff experienced constipation and a little trouble sleeping early, but both issues disappeared quickly. He says, "I wake up in the morning, and my fasting blood sugars are normal."
The medication took effect, he says, within 12 hours. He compared the feeling to having a gastric bypass.
"You cannot overeat food. As soon as you overeat, you almost feel ill." While it generally takes a few months to notice effects like A1C reduction and significant weight loss, side effects such as lower appetite may be felt immediately.
Weight loss was not his primary goal, but he lost about 35 pounds on the medication in the first five months. He also lost his sweet tooth. "I can maybe count three sweet things I have eaten since December."
Jeff found that his appetite slowly recovered days after taking tirzepatide. "You take the shot every Sunday, and by Saturday, you start to get a lot of appetite," he says. "It does not seem to affect your weight. If I eat a little bit more on Saturday night, on Sunday, the scale will not move one way or the other."
Jeff is allergic to hornets, so he already carries an auto-injector. He was not worried about using another drug delivered through a needle. "It's just a push button," he says. It also helped that his wife is a nurse. "So, I had her with me the first time to ensure I was doing it right. I didn't even feel it."
When Jeff was first prescribed tirzepatide, his insurance covered it. The company has since removed that benefit. He has filed an appeal but pays about $1,000 monthly out of pocket for his weekly injections. He plans to keep paying as long as necessary.
He considers the financial burden well worth it. "I have never had a medication that worked as well before for chronic conditions," Jeff says. "I've been blown away by it. For me, it's a miracle drug. It got rid of my diabetes" (4).

Self Quiz
Ask yourself...
- Can a provider willfully choose to prescribe tirzepatide before other diabetic medications are attempted?
- Would that impact his insurance coverage if Jeff did not meet the clinical criteria for using tirzepatide?
Use of Tirzepatide for Weight Loss Management
As mentioned, this medication is indicated for patients with a BMI of >30 or a BMI of >27 with qualifying comorbidities. Obesity can become a chronic lifetime disease, and for conditions such as these, the patient needs to implement therapy for the lifetime of the disease.
In a study conducted for tirzepatide, there was a dramatic increase in effectiveness compared to traditional nonsurgical interventions such as diet, exercise, and lifestyle changes. However, it has been noted that taking tirzepatide on an ongoing basis is recommended and necessary to maintain any weight loss achieved from the medication.
If a patient stops taking the drug, likely, it will no longer work (5).
Public health officials have expressed concerns about using the drug long-term. Still, data is currently lacking regarding long-term effectiveness, treatment duration, and maintaining weight reduction once the therapy is discontinued.
A recent trial consisted of 783 participants with a BMI greater than 30, and these participants agreed to take either a 10mg or 15mg dose of tirzepatide over 36 weeks. The injection is given once weekly, so this would equal a total of 36 injections.
By the end of 36 weeks, participants lost more than 21% of their body weight. After 36 weeks, participants continued on tirzepatide or received placebo treatment for the following year. The patients needed to be made aware of which treatment they were receiving.
Those still taking tirzepatide injections weekly after 88 weeks lost an additional 7% of their body weight, and those taking the placebo regained 15% at the end of 88 weeks (5).

Self Quiz
Ask yourself...
- What is the minimum BMI needed to qualify to receive this drug for weight loss management?
- Is this medication indicated for long-term use for patients with a high BMI?
Common Side Effects and Contraindications
Side Effects
Patients vary immensely with different experiences and side effects related to tirzepatide; however, the following are the most common side effects experienced by those taking the medication:
- Nausea
- Decreased appetite
- Vomiting
- Diarrhea
- Indigestion
- Constipation
- Stomach Pain
Tirzepatide usually does not cause fatigue, leaving one feeling weak, tired, and low energy. However, fatigue can be a common side effect of Type II diabetes.
It is important to note that most individuals who experience nausea, vomiting, and diarrhea episodes do so while the dosage increases, and typically, the symptoms decrease over time. G.I. effects were more prominent in those taking tirzepatide than those taking the placebo. The individuals not in the placebo group were more likely to stop treatment due to the unpleasant side effects (3).


Self Quiz
Ask yourself...
- Does tirzepatide cause fatigue in patients who use it?
Contraindications
Tirzepatide may cause thyroid tumors, including thyroid cancer, and it is essential to watch for possible symptoms, such as swelling or a lump in the neck, hoarseness, shortness of breath, or trouble swallowing.
Tirzepatide should also not be prescribed to any patient with Type 1 Diabetes.
One of the main ways that tirzepatide works is by stimulating the release of insulin from the pancreas, and due to this fact, there have not been many studies and clinical trials that include those with Type I diabetes.
However, this is not to say that prescribers have never ordered tirzepatide for those with Type I diabetes. Still, it is essential to note that if prescribed, it would be in addition to traditional insulin therapy.
- Personal or family history of a type of thyroid cancer known as medullary thyroid carcinoma (MTC).
- Any history of Multiple Endocrine Neoplasia syndrome type 2 (MEN 2).
- Patients who are allergic to the actual medication or any of its ingredients.
- Younger than 18 years of age


Self Quiz
Ask yourself...
- What is the reason that tripeptide is contraindicated in those with Type I diabetes?
- Why is there a risk with patients who have a thyroid disorder?
Safe Prescribing Practices, Guidelines, and Considerations for Providers
Safe Prescribing Practices
As with all prescribed medications, safe standards of care must be implemented and followed to ensure patient safety is maintained. The same applies to providers considering prescribing tirzepatide, and specific criteria must be met beforehand. The following information discusses guidelines involving exclusion and inclusion criteria for providers to prescribe tirzepatide (6) accurately.
Guidelines
Exclusion Criteria – If present, the following indicates that the patient should not receive tirzepatide:
- Diagnosis of Type I diabetes
- Personal or family history of medullary thyroid carcinoma or with Multiple Endocrine Neoplasia syndrome type 2
- Severe gastrointestinal dysmotility
- History of pancreatitis
- Pregnancy
- Proliferative Diabetic Retinopathy (PDR), severe Nonproliferative Diabetic Retinopathy (NDR), clinically significant myalgic encephalomyelitis (M.E.), or diabetic macular edema (DME) unless the risks/benefits have been discussed with the patient and are documented in the patient's health record along with monitoring plans and follow-up with an eye specialist who is informed at the time of initiation.
Inclusion Criteria – All of the following must be met for tirzepatide to be prescribed:
- Diagnosis of Type II diabetes
- A BMI of 25 or greater
- Inadequate glycemic control on at least 1mg of semaglutide injection plus two or more glucose-lowering drugs
- Change needed to achieve goal A1C is less than 1%.
- Goal A1C should be based on those recommended in the Diabetic Guidelines.
- Adherence to current diabetic medications as evidenced by a review of the prescription refill history during the six months.
Additional Inclusion Criteria – All of the following must be met for tirzepatide to be prescribed:
- Patients with atherosclerotic cardiovascular disease or chronic kidney disease
- Patients of childbearing potential who are using oral contraceptives
Inclusion Criteria for Weight Loss
- BMI of >30 or >27 with patient weight conditions.

Self Quiz
Ask yourself...
- Would a patient with a BMI of 23 with no comorbidities qualify to use tirzepatide to lose 5-10% of their body weight? Why not?
- What impact can tirzepatide have on a person with a healthy weight and BMI of <25?
Considerations for Providers
There are specific considerations that prescribers must be aware of when contemplating if a patient should receive the medication tirzepatide. The following is imperative and must be considered each time the medication is prescribed to a patient:
- Clinical Indications – indicated for treating adults with insufficiently controlled diabetes mellitus as an add-on therapy to diet and exercise; as monotherapy when metformin is considered inappropriate due to contraindications or intolerance; and other medicinal products for treating Type II diabetes.
- Monitoring of medication – routine monitoring of serum calcitonin or thyroid ultrasound is of uncertain value but is recommended for early detection of Medullary Thyroid Cancer (MTC).
- Cost – the average price for tirzepatide ranges from $1,071-$1,351 without any coupons or insurance. Savings Card – manufacturer provided; patients can pay as little as $25 monthly for up to 12 injections. Savings Card – manufacturer provided; patients can pay as little as $25 monthly for up to 12 injections.
- Benefits and Risks – One must evaluate the effectiveness of diabetes and the weight loss experienced. Some of the risks must be evaluated, such as increased cost of medication, unpleasant gastrointestinal side effects, poor insurance coverage, and drug shortages. The FDA has warned that the medicine can cause thyroid C-cell tumors in rats, and it is not sure whether tirzepatide causes similar tumors.
How long does it take for tirzepatide to begin working?
Tirzepatide will start to lower one's blood sugar levels immediately, but it can take 8 to 12 weeks to reach one's target A1C goal.
Compared to other diabetic treatments, studies have shown that it can take eight weeks to reach an A1C target of less than or equal to 7% and 12 weeks to get an A1C of less than or equal to 6.5%. Significant weight loss can occur as early as 28 weeks.
Safe Administration
It is essential to follow the correct steps for safe administration of tirzepatide as listed below:
- The recommended starting dosage is 2.5mg, injected subcutaneously once weekly. The 2.5mg dosage is for treatment initiation and not for glycemic control.
- After four weeks, increase the dosage to 5mg, injected subcutaneously once weekly.
- If additional glycemic control is needed, increase the dosage in 2.5mg increments after at least four weeks on the current dose.
- The maximum dosage is 15mg, injected subcutaneously once weekly.
- If a dose is missed, instruct patients to administer it as soon as possible, within four days (96 hours) after the missed dose. If more than four days have passed, skip the missed dose, and administer the next dose on the regularly scheduled day. In each case, patients can then resume their regular once-weekly dosing schedule.
- The day of weekly Administration can be changed, if necessary, as long as the time between the two doses is at least three days (72 hours).
- Before initiation, train patients and caregivers on proper injection techniques.
- Instruct patients using the single-dose vial to use a syringe appropriate for dose administration (e.g., a 1ml syringe capable of measuring a 0.5 mL dose).
- Administer the medication once weekly, any time of day.
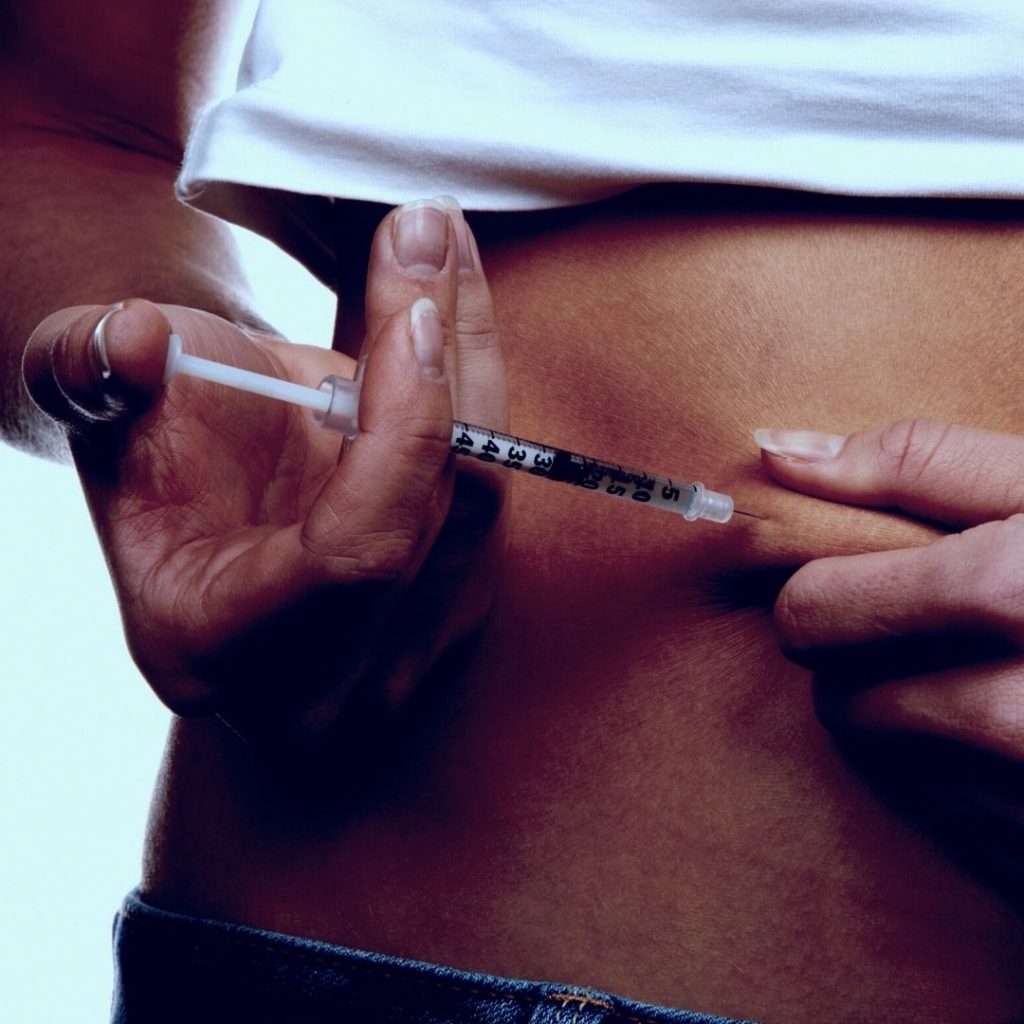
- Inject the medication subcutaneously in the abdomen, thigh, or upper arm.
- Rotate injection sites with each dose.
- Inspect the medication visually before use. It should appear clear and colorless to slightly yellow. Do not use the medicine if particulate matter or discoloration is seen.
- When using the medication with insulin, administer it as separate injections and never mix. It is acceptable to inject tirzepatide and insulin in the same body region, but the injections should not be adjacent.
Does the tirzepatide injection hurt when administered?
Pain from the injection site has not been reported as a common side effect, but it may occur.
Due to the injection being given subcutaneously, slight pain or discomfort can occur.

Self Quiz
Ask yourself...
- The patient asks you," How long will this take to work?" How will you respond?
- The patient reports they have never used an injection before; what methods can you use to teach your patient how to administer this medication safely?
Alternatives to Tirzepatide for Weight Loss Management
In some instances, patients need to be aware of alternatives to tirzepatide in case they cannot take the actual injection for whatever reason. In cases such as these, there are alternative supplements that can be purchased over the counter, and they include the following (7):
- PhenQ – top OTC choice – comprehensive weight loss solution that targets specific body regions, facilitates prompt fat loss, and expedites the weight loss journey.
- PhenGold – the most potent OTC weight loss alternative – one of the top weight loss supplements that boost metabolism, making one less hungry, less tired, and an overall improved feeling.
- Capsiplex BURN – the best choice for men – helps to burn fat faster and keep blood sugar levels in check. It helps to keep one's muscles, curbs hunger, gives one more energy, and torches stubborn fats.
- Trimtone – the best choice for women – helps women to lose weight, eat less, increase metabolism, burn extra calories, and boost energy.
- Prime Shred – best fat burner for men – boosts metabolism, keeps muscles intact, increases energy, and helps maintain focus.
The advanced practicing nurse or prescriber needs to inform patients about alternative options such as these in an effort for individuals to understand that other choices are available and can be used. Many individuals need to be more knowledgeable about alternatives besides tirzepatide due to the extra hype from social media sources that promote advertisements related to tirzepatide only but do not mention the other options.
Why does social media influence and encourage patients to take tirzepatide?
Social media trends can be helpful but can also become harmful by setting unrealistic expectations and promoting a diet culture mentality. They can create an unhealthy obsession with "clean" eating, especially in the younger populations.
Due to this, many individuals take the medication despite any occurrence or history of Type II diabetes, and the drug can ultimately become misused.
It has been noted that there is an influx of patients requesting this medication for weight loss instead of the intended purpose, which is to help control Type II diabetes.
Tirzepatide represents one of the most recent non-medical treatments aimed at managing the symptoms of Type II diabetes. While it is not indicated for weight management, diabetic patients who receive it frequently report a significant reduction in body weight.
Empirical evidence suggests the efficacy of tirzepatide in weight management, and certain physicians currently endorse the Administration of the medication as a therapeutic and effective means to overcome obesity.
What are some severe side effects of tirzepatide that can impact patient safety?
The Administration of tirzepatide can benefit many individuals, but some severe side effects must be mentioned.
These include thyroid tumors, thyroid cancer, pancreatitis, hypoglycemia, serious allergic reactions, kidney issues, severe stomach problems, vision changes, and gallbladder issues. All these side effects must be taken seriously and reported, as they can lead to life-threatening

Self Quiz
Ask yourself...
- With what you have learned in this course, what education will you provide to patients requesting this medication for weight loss?
- Have you seen increased demand for this medication in your current practice?
- If you Google tirzepatide, your results will likely include links to telehealth services promoting this weight-loss medication. To determine eligibility, what special considerations need to be taken to assess a telehealth patient?
Conclusion
Medications like tirzepatide are game changers for those patients with type 2 diabetes that have failed other medications. Unfortunately, several companies seek to profit from its weight-loss benefits through aggressive marketing campaigns that limit the available supply and increase the costs for those who need it. As healthcare providers, we need to use sound clinical judgment and follow the exclusion/inclusion criteria and other guidelines before prescribing this medication, so we do not unintentionally cause harm while looking to appease our patients who request this.
References + Disclaimer
- Katie Kerwin McCrimmon, Uch. (2023, May 15). What is Mounjaro? and does it work better for weight loss than Ozempic and Wegovy?. UCHealth Today. https://www.uchealth.org/today/what-is-mounjaro-and-how-does-it-work-for-weight-loss/
- National Institutes of Health. (2023, July 28). DailyMed – mounjaro- tirzepatide injection, solution. U.S. National Library of Medicine. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d2d7da5d-ad07-4228-955f-cf7e355c8cc0
- Type 2 Diabetes Treatment to Lower A1C | Mounjaro® (tirzepatide). (2023). https://www.mounjaro.com/?utm_id=go_cmp-16966473997_adg-133042365262_ad-626584063672_kwd-1651326559888_dev-c_ext-_prd-_mca-_sig-EAIaIQobChMIxcXZqYeHgQMVhDjUAR2Lvw5CEAAYASAAEgIMkfD_BwE&utm_source=google&utm_medium=ppc&campaign=16966473997&adgroup=133042365262&ad=626584063672&utm_keyword=kwd-1651326559888&gclid=EAIaIQobChMIxcXZqYeHgQMVhDjUAR2Lvw5CEAAYASAAEgIMkfD_BwE
- GoodRX – error. (2023, July 14). https://www.goodrx.com/mounjaro/how-make-you-feel
- Chen, S. (2023, July 28). Diabetes drug Mounjaro shown to have extraordinary weight loss for people without diabetes. diaTribe. https://diatribe.org/diabetes-drug-mounjaro-shown-have-extraordinary-weight-loss-people-without-diabetes
- VA Pharmacy Benefits Management Services. (2022, August). Tirzepatide Injection Criteria for Use. https://www.va.gov/formularyadvisor/DOC_PDF/CFU_Tirzepatide_MOUNJARO_Criteria_Aug_2022.pdf.
- Allied PR. (2023 B.C.E., May 6). Mounjaro Weight Loss: Newly Launch Over The Counter Alternative Pills To Tirzepatide & Mounjaro Weight Loss. https://newsdirect.com/news/mounjaro-weight-loss-newly-launch-over-the-counter-alternative-pills-to-tirzepatide-and-mounjaro-weight-loss-300908569. Retrieved August 30, 2023, from https://newsdirect.com/news/mounjaro-weight-loss-newly-launch-over-the-counter-alternative-pills-to-tirzepatide-and-mounjaro-weight-loss-300908569
- Mounjaro (2023). Savings and support for Mounjaro. Retrieved on August 31st 2023 from https://www.mounjaro.com/savings-resources?gclid=Cj0KCQjw9MCnBhCYARIsAB1WQVWAcINPKFwiHl4SbZQiGC8E_o9wHVOkDJDDUuphW3OlsKBagVTF1rQaApLJEALw_wcB
Disclaimer:
Use of Course Content. The courses provided by NCC are based on industry knowledge and input from professional nurses, experts, practitioners, and other individuals and institutions. The information presented in this course is intended solely for the use of healthcare professionals taking this course, for credit, from NCC. The information is designed to assist healthcare professionals, including nurses, in addressing issues associated with healthcare. The information provided in this course is general in nature and is not designed to address any specific situation. This publication in no way absolves facilities of their responsibility for the appropriate orientation of healthcare professionals. Hospitals or other organizations using this publication as a part of their own orientation processes should review the contents of this publication to ensure accuracy and compliance before using this publication. Knowledge, procedures or insight gained from the Student in the course of taking classes provided by NCC may be used at the Student’s discretion during their course of work or otherwise in a professional capacity. The Student understands and agrees that NCC shall not be held liable for any acts, errors, advice or omissions provided by the Student based on knowledge or advice acquired by NCC. The Student is solely responsible for his/her own actions, even if information and/or education was acquired from a NCC course pertaining to that action or actions. By clicking “complete” you are agreeing to these terms of use.
➁ Complete Survey
Give us your thoughts and feedback
➂ Click the Green MARK COMPLETE Button Below
To receive your certificate
