The Shingrix Vaccine
GlaxoSmithKline (GSK) launched the Shingrix vaccine in 2017, with better-than-expected performance. The company experienced supply shortages because of higher-than-expected demand. Through March 2020, 17 million people had received the Shingrix® vaccine, becoming the preferred vaccination against shingles.
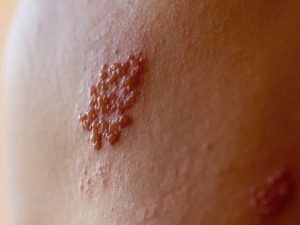
Unlike the influenza vaccine, Shingrix is not a seasonal vaccination. Per recommendations, the vaccine is the current best method to prevent herpes zoster reactivation. Shingrix is a recombinant zoster vaccine (RZV) designed to provoke an immune response and proven safe and encouraged within the ongoing pandemic.
No contraindications exist with the Shingrix vaccine in people recovered from COVID-19, as long active infection has subsided.
Active shingles infection offers no herd protection, negating the need for factors of social distancing or risk of disease transmission. Shingrix, an inactive vaccine, needs to be considered in the immunosuppressed population.
Individuals aged 19 and older who are immunodeficient or immunosuppressed secondary to disease or therapy are now eligible for the vaccine. Who should not get the vaccine? Pregnant individuals need to wait to get the Shingrix vaccine. The earlier vaccine, Zostavax was a live, weakened virus. Shingrix provides strong efficacy in individuals age 50 and older.